1. Introduction
Neurodevelopmental damage resulting from fetal alcohol exposure is a preventable but leading cause of severe intellectual disability in the United States and abroad [1–4]. Fetal alcohol spectrum disorders (FASDs) are the result of the teratogenic effects of alcohol on the developing nervous system, resulting in effects that range from decreases in cortical thickness [5,6], brain volume and neural activity [6–11], to impaired development of brain regions such as the basal ganglia, cerebellum, hippocampus, and prefrontal cortex [7,8,12,13], to disruptions in learning and memory associated with mild developmental alcohol exposure [14]. In addition to impaired performance in school or on academic achievement tests, prenatal alcohol exposure impairs performance on laboratory tasks, including eyeblink conditioning [15–17], spatial recognition, and working memory [10,18–20]. Recent conservative estimates of FASD prevalence place it as high as 5% in diverse US communities, which underscores it as a serious and unanswered societal problem [4].
Animal models of FASD have been instrumental in isolating the neural and behavioral disruptions caused by developmental alcohol exposure because of the ability to manipulate multiple factors such as exposure window, pattern, and dosage [21]. In rats, neonatal ethanol exposure during the brain growth spurt (i.e., PD4–9, roughly equivalent to the third trimester of human pregnancy) captures many aspects of impaired brain and behavior similar to the human condition [2,15,21,22]. Indeed, binge-like ethanol exposure during this period severely disrupts neuronal and molecular signaling in the hippocampus in a manner that cannot be fully attributed to CA1 pyramidal cell loss [23–27]. This exposure disrupts activity-and plasticity-associated gene expression, neuroplasticity (i.e., long-term potentiation [LTP]), as well as cholinergic and glutamatergic receptor composition and signaling in the hippocampus [24,25,28–32]. In contrast, this exposure has no significant effect on prefrontal cell number but instead alters prefrontal dendritic complexity, DNA methylation, and neurophysiological spine properties [33–35]. These few studies have not addressed the fundamental question of how subtle alterations in prefrontal molecular neurobiology and function affect learning and memory engaging the prefrontal cortex in animal models of FASD.
Our lab has shown that a variant of Pavlovian contextual fear conditioning, called the Context Preexposure Facilitation Effect (CPFE), is particularly sensitive to the effects of developmental alcohol exposure in rats [24,27,36–39]. In the CPFE, learning about the context, acquiring a context-shock association, and retrieval of contextual fear is separated into three distinct phases each separated by 24hr - context preexposure, immediate-shock training, and retention testing. During context preexposure, animals encode the features of the training context that are subsequently consolidated into a conjunctive context representation [40–42]. During immediate-shock training, the conjunctive context representation is retrieved via pattern completion and is subsequently associated with an immediate foot-shock. The presence of post-shock freezing immediately after conditioning reflects successful acquisition of the context-shock association, whereas retention test freezing reflects successful consolidation and later retrieval of this association. Importantly, our lab has recently shown that both the dorsal hippocampus (dHPC) and medial prefrontal cortex (mPFC) are required during all three phases of the CPFE [43–45]. Additionally, context preexposure and immediate-shock training induces the expression of the activity- and plasticity-associated immediate early genes (IEGs) c-Fos, Arc, Npas4, and Egr-1 in the mPFC and dHPC [46,47]. These combined neural and behavioral aspects of the CPFE may explain why it is more sensitive to neonatal alcohol exposure than traditional “hippocampus-dependent” behavioral tasks such as single-trial standard contextual fear conditioning (sCFC) or maze-learning tasks commonly used in FASD models [24,36]. Indeed, neonatal ethanol exposure (from PD4–9 or PD7–9) completely abolishes retention test freezing in the CPFE in a dose-dependent fashion [24,36,37,39]. Despite this, the specific phase of the CPFE that is disrupted by PD4–9 ethanol exposure and the role of altered prefrontal signaling in this impairment is currently unknown.
The purpose of the current study was to address this question and to characterize the role of altered prefrontal molecular signaling in alcohol-induced disruption of the CPFE. Based on our recent findings demonstrating a necessary role of the mPFC in context memory [43,44], we hypothesized that PD4–9 ethanol exposure would result in impaired context learning as well as disrupted prefrontal and hippocampal IEG expression on the preexposure day of the CPFE. Furthermore, we hypothesized that this disruption would result in reduced or abolished post-shock freezing in the CPFE, as alcohol-exposed animals will have encoded or consolidated a weak context representation during context preexposure. While there is some evidence that prenatal alcohol exposure impairs prefrontal IEG expression [48,49], to our knowledge, this is the first study to examine behaviorally-driven expression of multiple activity- and plasticity-associated IEGs in the mPFC after neonatal ethanol exposure in a rat model of FASD.
2. Materials and Methods
2.1. Subjects
Animal husbandry was as described in our previous report [43,46,50]. Across the three experiments, there were a total of 250 adolescent (PD31) Long Evans rats (131 females and 119 males), derived from 36 separate litters bred by the Office of Laboratory Animal Medicine at the University of Delaware. The animal housing facility was maintained on a 12:12 h light/dark cycle with lights on at 7:00 am. Litters were culled on PD3 to eight pups (4 males and 4 females when possible), and pups were weaned from their mother on PD21 and housed with same-sex littermates. On PD29 rats were individually housed in small clear cages with ad libitum access to water and rat chow for the remainder of the experiments (until PD33–34). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Delaware following guidelines established by the National Institute of Health.
2.2. Neonatal alcohol dosing (PD4–9, 5.25g/kg/d split into two daily doses)
Neonatal ethanol dosing via intragastric intubation occurred over PD4-PD9 with methods that have been described previously [37,51]. Littermates were randomly assigned to receive either ethanol (Group EtOH) or sham (Group SI) intubations, with an equal number of males and females in each litter whenever possible. Same-sex littermates assigned to the same dosing condition (EtOH or SI) were assigned to different behavioral groups so that no more than one same-sex littermate was assigned to any particular experimental condition. Briefly, on PD4, pups were separated from their mothers and placed into weigh boats set over a heating pad that provided warmth during the separation. Pups were weighed prior to the first intubation session (occurring daily at 9am ± 1hr). The intubation process involved passing PE10 tubing lubricated with corn oil down the esophagus and into the stomach of the rat pup. Animals in the SI group received intragastric intubations on the same schedule as the EtOH group, and the tube was removed after approximately 6–8 seconds during each scheduled intubation without the infusion of any solution. Animals in the EtOH group were intubated and given a daily dose of 5.25 g/kg of alcohol, [11.9% v/v ethanol (made from 95% ethanol)] in a custom milk formula previously described [52]. This dose was divided into two feedings each day, separated by 2hr. The formula was delivered in a volume of 0.02778 ml ⁄g body weight. A third intubation of the milk formula (containing no ethanol) was administered two hours after the second daily alcohol dosing. After each intubation was completed (<20 minutes per litter), pups were returned as a litter to their mothers.
2.3. Blood alcohol concentrations (BACs)
On PD4, 90 min following the second alcohol intubation, pups received a small tail-clip and a 20μl blood sample was collected using a capillary tube. Blood samples from Group SI were discarded and those from alcohol-exposed pups were saved for further blood alcohol analysis. Blood samples from alcohol-exposed pups were centrifuged, and the plasma was collected and stored at −20°C. Blood alcohol concentrations were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described [36]. Briefly, the rate of oxidation of alcohol in each plasma sample was measured. BACs (expressed in mg⁄dl) were calculated based on comparisons to known values of an alcohol standard solution.
2.4. Apparatus and stimuli
The apparatus and stimuli used have been described previously [37,46]. Briefly, fear conditioning occurred in four clear Plexiglas chambers arranged in a 2 × 2 formation on a Plexiglas stand within a fume hood (Context A – Pre Group). Each chamber had a grid floor made of nine stainless steel bars connected to a shock scrambler (Med Associates, Georgia, VT-ENV-414S). The alternate context (Context B – Alt-Pre Group) consisted of the same Plexiglas chambers with a convex wire mesh insert that covered the back wall and floor of the chamber and a white paper sleeve that covered the outside walls of the chamber. In Experiment 1B, 2, and 3, the unconditioned stimulus (US) was two, 1.5 mA foot-shocks, each 2s in duration, and presented 1s apart immediately upon chamber entry. In Experiment 1A, one immediate shock was given instead of two. Videos of each session (preexposure, training, testing) were recorded using Freeze-Frame 3.0 software (Actimetrics, Wilmette IL) with freezing defined as a bout of 0.75 s or longer without a change in video pixilation (see section 2.8.2).
2.5. Behavioral procedures
2.5.1. Experiment 1 - Context Preexposure Facilitation Effect (CPFE)
The CPFE procedure used in Experiment 1 took place over the course of three days from PD31 to PD33 and has been described previously [43,46,50]. Rats were assigned to either preexposure condition (Pre group), alternate preexposure condition (Alt-Pre group). Animals in the Pre group received exposure to Context A, the training context, while animals in the Alt-Pre group received exposure to Context B (see section 2.4). Alt-Pre animals serve as non-associative behavioral controls as they demonstrate the immediate-shock deficit (ISD), which reflects an inability to form a context-shock association without prior exposure to Context A [53].
On PD31, rats were placed in Context A or B and underwent multiple context preexposure, consisting of one initial 5 min exposure to the chamber, followed by five 1 min exposures, with a 1 min interval between exposures. On PD32, single rats were carried into the testing room, placed in their respective Context A training chamber, and within 3s, were given one 1.5 mA 2s foot-shock (Experiment 1A) or two (Experiment 1B) foot-shocks separated by 1s. In both Experiments 1A and 1B, foot-shock was followed by a 3-min post-shock freezing test (with no additional US presentations). On PD33, rats were returned to the same Context A chamber in which they were trained for a 5min retention freezing test.
2.5.2. Experiment 2 - Standard contextual fear conditioning (sCFC)
The sCFC procedure used in Experiment 2 has been described previously [43]. The sCFC procedure took place over the course of two days from PD31 to PD32. All chambers, stimuli, and drug infusion protocols used were identical to the ones used in Context A for the CPFE experiments (see section 2.4). On PD31, animals were assigned to either the Delayed-shock or Imm-Shock control condition. Animals in the Delayed-shock condition received three minutes of context exposure in Context A, followed by two 1.5 mA 2s foot-shocks separated by 1s. Animals in the Imm-Shock conditions were given two foot-shocks without any context exposure. This group served as behavioral controls for the delayed-shock conditions as the placement-to-shock interval was under 5 sec resulting in the ISD (Fanselow, 1990). Rats were removed immediately after conditioning without any post-shock freezing test. On PD32, rats in both retention conditions were tested in Context A for 5min in the same chamber in which training occurred.
2.5.3. Experiment 3 – CPFE
The CPFE procedure used in Experiment 3 was identical to Experiment 1B (i.e., using 2 immediate shocks on the training day). Rats were assigned to either preexposure condition (Pre group), alternate preexposure condition (Alt-Pre group), or a behaviorally naïve home-cage condition (HC) that serves to establish baseline gene expression. In Experiment 3, SI and EtOH animals in the Pre group were sacrificed via live decapitation and tissue was collected for RNA extraction and qPCR 30min after context preexposure on PD31 (see sections 2.6 and 2.7). A subset of rats from each litter and training cohort served as a behavior group and underwent the full 3-day CPFE procedure without any tissue collection to provide behaviorally-tested counterparts to the rats used for IEG analysis. Notably, the current study chose not to sacrifice Alt-Pre animals in the IEG design in Experiment 3 as there is no difference between Pre and Alt-Pre gene expression on the preexposure day of the CPFE [46].
2.6. Brain removal and tissue dissections
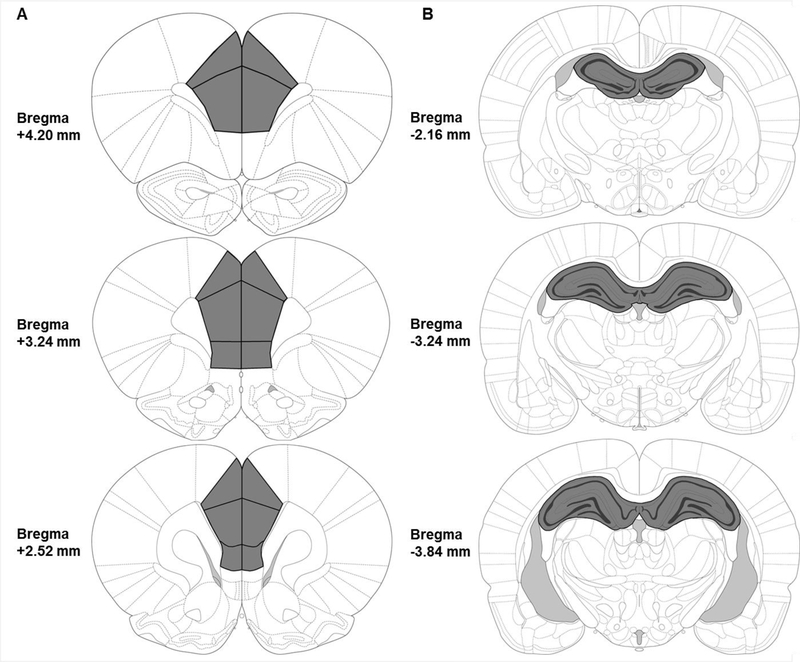
In Experiment 3, thirty minutes after context preexposure in Context A, rats were rapidly decapitated without anesthesia. Brains were removed and dropped into ice-cold saline for 10 seconds to increase tissue firmness. Coronal brain slabs (1–1.5 mm) were cut out of the whole brain using a young adult rat brain matrix. The mPFC and dHPC were dissected out of the coronal slabs, checking each side of the slab to ensure that dissection was not too anterior or posterior to the targeted brain regions. As in a previous study [46], the boundaries of dissection were +4.20mm to +2.52mm from bregma for the mPFC, and −2.16mm to −3.84mm from bregma for the dHPC (see Figure 4). Dissected tissue was immediately flash frozen on dry ice and subsequently stored at −80 °C until the time of analysis. Dissector identity was counterbalanced across animal litter, sex, and experimental condition.
Figure 4.
Illustration of brain regions analyzed (A, Left: mPFC; B, Right: dHPC), with dissected regions outlined in black and shaded in dark gray. Tissue from the mPFC was collected between approximately +4.20 mm to +2.52 mm relative to bregma; tissue from the dHPC was collected between about −2.16 mm to −3.84 mm relative to bregma. Images are adapted from The Rat Brain in Stereotaxic Coordinates, 6th Ed (Paxinos & Watson, 2007).
2.7. Quantitative Real-time PCR
The quantitative real-time PCR procedure used has been described previously [46,55]. RNA was extracted from frozen tissue samples using TRIzol Reagent (Cat. No. 15596018, Invitrogen). Genomic DNA was eliminated and cDNA was synthesized from extracted RNA (1000ng/μL) using the QuantiTect® Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was quantified by real-time PCR using the GREEN FASTMIX PERFECTA-SYBR Kit (Cat. No. 101414–270, Quantabio) in 10μL reactions on a CFX96Touch real time PCR machine. Expression of Egr-1 was analyzed using a QuantiTect® Primer Assay (Cat. No. QT00182896, Qiagen) and diluted according to protocol. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 μM (18s, Arc, c-Fos, and Npas-4). 18s is a ribosomal housekeeping gene and was used as a control gene for all experimental groups as it did not differ significantly across any groups or manipulations. Samples were numbered, blinded to treatment group and run in duplicate on real-time PCR plates. For each reaction, the average quantitative threshold amplification cycle number (Cq) value was determined from each duplicate, and the 2-ΔΔCq method was used to calculate the relative gene expression for each gene of interest relative to the control gene.
2.8. Data analysis and statistics
2.8.1. Analysis of body weight
Neonatal body weight was analyzed with a repeated measures ANOVA with a between-subjects factor of dosing condition (SI vs. EtOH) and the within-subjects factor of age (PD4 vs. PD9). Analyses of neonatal body weight was collapsed across both experiments, as there was no main effect or interaction as a function of experiment (ps > .50). There were also no main effects or interactions involving sex in the PD4 and PD9 weights (ps > .40) so the data were collapsed across this variable at these ages. Body weight at PD31 was analyzed with a 2 (Sex; male vs. female) × 2 (Dosing condition; SI vs. EtOH) factorial ANOVA. Body weight averages (PD4, PD9, PD31 males and females) and BACs for both dosing conditions appear in Table 1.
Table 1.
Body weights and BACs for Experiments 1A, 1B, and 2. Average body weights (in grams ± SE) are given from the SI and EtOH groups at the first and last day of the dosing period (PD4 and PD9, respectively) and the first day of behavioral training (PD31). BACs (in mg/dl ± SE) were taken from blood samples collected on PD4 from the EtOH group.
| Experiment | Dose | n = | Body weight (Grams ± SEM) | PD4 BACs (mg/dl) | |||
|---|---|---|---|---|---|---|---|
| PD4 | PD9 | PD31 (males) | PD31 (females) | ||||
| Experiment 1A | SI | 30 | 10.89 ± 0.17 | 20.48 ± 0.35 | 111.25 ± 2.6 | 99.07 ± 0.92 | N/A |
| CPFE 1-shock | EtOH | 21 | 10.86 ± 0.19 | 17.54 ± 1.55* | 105.7 ± 2.45 | 97.64 ± 1.06 | 428.85 ± 10.21 |
| Experiment 1B | SI | 33 | 11.494 ± 0.2 | 20.75 ± 0.35 | 109.77 ± 2.68 | 100.3 ± 0.96 | N/A |
| CPFE 2-shock | EtOH | 33 | 11.34 ± 0.2 | 18.794 ± 0.34* | 110.8 ± 1.43 | 98.67 ± 1.03 | 417.48 ± 7.40 |
| Experiment 2 | SI | 18 | 11.72 ± 0.26 | 21.08 ± 0.44 | 125.12 ± 2.8 | 110.7 ± 2.12 | N/A |
| sCFC | EtOH | 15 | 11.85 ± 0.23 | 17.44 ± 0.52* | 118.1 ± 3.34 | 108.44 ± 2.17 | 407.05 ± 7.04 |
| Experiment 3 | SI | 55 | 10.92 ± 0.13 | 20.18 ± 0.33 | 112.07 ± 1.78 | 99.43 ± 0.70 | N/A |
| CPFE Pre day IEG | EtOH | 45 | 11.24 ± 0.18 | 17.74 ± 0.25* | 108.86 ± 2.04 | 98.35 ± 1.05 | 423.11 ± 7.45 |
| Collapsed | SI | 137 | 11.16 ± 0.09 | 20.50 ± 0.19 | 113.03 ± 1.29 | 101.17 ± 0.07 | N/A |
| Expts.1–3 | EtOH | 115 | 11.29 ± 0.11 | 17.89 ± 0.17* | 110.1 ± 1.41 | 99.80 ± 1.61 | 420.31 ± 4.69 |
indicates a significant difference between the SI and EtOH groups.
2.8.2. Analysis of behavioral data
Behavioral data processing procedures have been described previously [46]. A human observer blind to the experimental groups verified the freezing threshold setting with Freeze View 3.0 (Actimetrics, Wilmette IL). The software program computes a “motion index” that was adjusted to set a freezing threshold separately for each animal (per software instructions) by a blind observer who verified from the video record whether or not small movements were scored as freezing. Once set, the threshold did not change during a session. We have validated this procedure against other scoring methods (e.g., hand scoring of video records by two blind observers). Freezing behavior was scored as the total percent time spent freezing longer than .75s bins (defined as the cessation of all movement except breathing) in each respective session bin (context exposure, post-shock freezing, and a 24 h retention test). The data were imported into STATISTICA 64 data analysis software and freezing behavior was analyzed. There were no main effects or interactions involving sex on freezing behavior across any of the experiments (ps > .20), so the data were collapsed across this variable. In Experiments 1 and 3, Post-shock and retention test freezing data were analyzed using 2 (Dosing condition; SI vs. EtOH) × 2 (Exposure condition; Pre vs. Alt-Pre) × 2 (within subjects; Phase of testing; Post-shock vs. Retention) repeated measures ANOVAs. In Experiment 2, the immediate shock control group was pooled across dosing condition as there was no significant difference between to two groups and they froze uniformly low (p > .50). Retention test freezing data were analyzed using a one-way ANOVA (EtOH-Delayed vs. SI-Delayed vs. Pooled-ImmShock). Post-hoc contrasts were performed with Newman–Keuls tests. Rats were excluded from analysis as an outlier if they had a score of ± 1.96 standard deviations from the group mean, however, the average Z-score of removed outliers averaged across all experiments was ± 5.45 (± 1.04 SEM). One animal from each group (EtOH-Alt-Pre, EtOH-Pre, SI-Alt-Pre, and SI-Pre in Experiments 1 and 3; SI-Delayed and EtOH-Delayed in Experiment 2) was excluded as an outlier from both the post-shock and retention freezing data in each experiment.
2.8.3. Analysis of qPCR data
Relative gene expression for the IEGs c-Fos, Arc, Egr-1, and Npas4 in the mPFC and dHPC was determined in Experiment 3 (see section 2.7). The relative gene expression value was obtained by normalizing the data to the reference gene (18s) and to the home-cage control group average delta CT for each gene in each experiment [46]. Consistent with previous findings [46], there were no interactions involving sex across any of the experiments (ps > .30), so the data were collapsed across this variable. There was also no difference between the raw data in HC group dosed with alcohol or sham-intubated, so the HC group was collapsed across dosing condition (ps > .20). Gene expression in Experiment 3 was analyzed using a one-way ANOVA (HC, SI-Pre, and EtOH-Pre) for each gene (c-Fos, Arc, Egr-1, and Npas4) in both the mPFC and dHPC. Post-hoc contrasts were performed with Newman–Keuls tests. The number of outliers removed in each sampling condition in Experiment 3 can be found in Table 2. The average Z-score of removed outliers was ± 3.22 (± 0.22 SEM).
Table 2.
Final group numbers (n), number of outliers removed (HC, Alt-Pre, Pre), and statistical results for all one-way ANOVAs (see F and p values) for each gene (c-Fos, Arc, Egr-1, and Npas4) in each region (mPFC and dHPC) for Experiment 2.
| Experiment 3: CPFE Preexposure Day IEG Expression (Pre Group) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medial prefrontal cortex (mPFC) | Dorsal hippocampus (dHPC) | |||||||
| Genes | F | p | n (HC, EtOH, SI) | Outliers (HC, EtOH, SI) | F | p | n (HC, EtOH, SI) | Outliers (HC, EtOH, SI) |
| c-Fos | 73.81 | < .0001 | 20, 10, 11 | 2, 1, 1 | 25.32 | < .0001 | 20, 11, 10 | 2, 1, 1 |
| Arc | 45.36 | < .0001 | 19, 10, 11 | 2, 1, 1 | 20.75 | < .0001 | 20, 11, 10 | 2, 1, 1 |
| Egr-1 | 6.62 | < .001 | 20, 10, 11 | 2, 1, 1 | 1.14 | > .30 | 20, 11, 10 | 2, 1, 1 |
| Npas4 | 33.7 | < .0001 | 21, 10, 11 | 1, 1, 1 | 19.87 | < .0001 | 20, 11, 11 | 2, 1, 0 |
3. Results
3.1. Body weight and BACs (all experiments)
Body weight averages for sham-intubated and alcohol-exposed animals at PD4, PD9, and PD31 appear in Table 1. Both the SI and EtOH groups gained substantial weight during the dosing period (PD4-PD9) up until the age of testing (PD31). A 2 (Dosing condition; SI vs. EtOH) × 2 (Age; PD4 vs. PD9) repeated measures ANOVA revealed significant main effects of Dosing condition [F(1, 250) = 45.10, p < .001], Age [F(1, 250) = 5372.73, p < .001], as well as a Dosing condition × Age interaction [F(1, 250) = 156.90, p < .001]. Newman-Keuls tests revealed no difference between group weights on PD4 (ps > .50), but on PD9, EtOH animals weighed about 13% less than SI animals (ps < .001). Transient growth retardation in ethanol treated animals over this dosing period has been reported previously [24,27,37,56]. Ethanol did not alter body weight at the time of testing. A 2 (Dosing condition; SI vs. EtOH) × 2 (Sex; male vs. female) factorial ANOVA performed on PD31 body weights revealed a significant main effect of Sex [F(1, 248) = 111.45, p < .001] but not Dosing condition [F(1, 248) = 3.86, p > .05], with no interaction between these two variables [F(1, 248) = 0.41, p > .50]. Females had reduced body weights compared to males at PD31 regardless of dosing condition (see Table 1).
BACs taken from the blood samples of the EtOH group on PD4 (see section 2.3) are also shown in Table 1 (grouped by experiment and then collapsed across all experiments). The EtOH group showed an average BAC of 422.45 ± 4.69 mg/dl. There was no significant effect of experiment (1A vs. 1B vs. 2 vs. 3) or sex (male vs. female) on BACs (ps > .30).
3.2. Experiment 1A: PD4–9 ethanol exposure abolishes post-shock and retention test freezing (1-shock reinforcement)
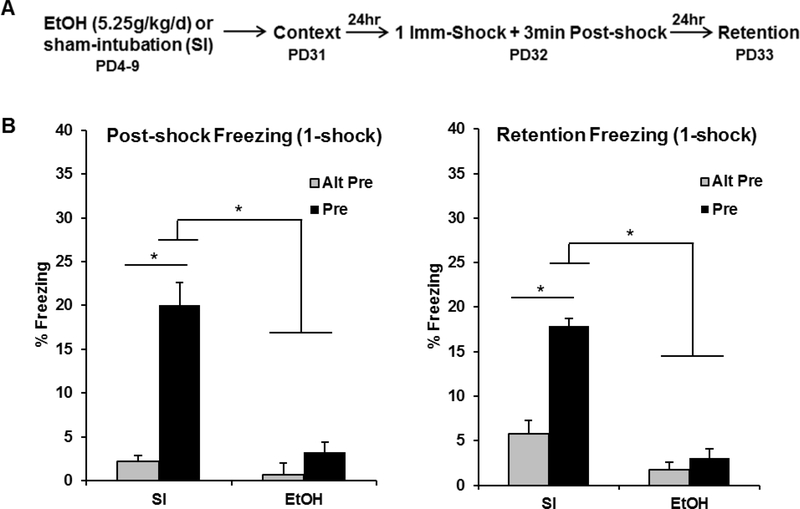
The purpose of Experiment 1A was to examine the effects of PD4–9 ethanol exposure on post-shock and retention test freezing in the CPFE. The behavioral design and results for Experiment 1A can be seen in Figure 1. Analyses for Experiment 1A were run on 47 animals distributed across the following groups: EtOH-Alt-Pre (n=7), EtOH-Pre (n=12), SI-Alt-Pre (n=13), and SI-Pre (n=15). Repeated measures ANOVA revealed a significant main effect of Dosing [F(1, 41) = 55.35, p < .001], Exposure [F(1, 41) = 45.92, p < .001], and a significant Dosing × Exposure interaction [F(1, 41) = 26.98, p < .001]. There was no main effect or any interactions involving Phase (ps > .08). The SI-Pre group froze significantly more than all other groups during both the post-shock and retention freezing tests (ps < .001). There was no difference between EtOH animals preexposed to the training context (EtOH-Pre) and non-associative controls preexposed to an alternate context (EtOH-Alt-Pre or SI-Alt-Pre; ps > .50) in either phase. These results show that PD4–9 ethanol exposure abolishes post-shock and retention test freezing in the CPFE.
Figure 1.
Behavioral design (A) and mean percent freezing (± SEM) for the 3min post-shock (B) or 5min retention (C) freezing tests. (A) Animals were given alcohol or sham-intubation from PD4–9, and then run through the full three-day CPFE procedure from PD31–33. The US was one immediate shock. (B, C) The SI-Pre group froze significantly higher than the EtOH-Pre group and both Alt-Pre control groups during the 3min post-shock and 5min retention freezing tests (ps < .001). * indicates p < .001
3.3. Experiment 1B: PD4–9 ethanol exposure impairs post-shock and abolishes retention test freezing (2-shock reinforcement)
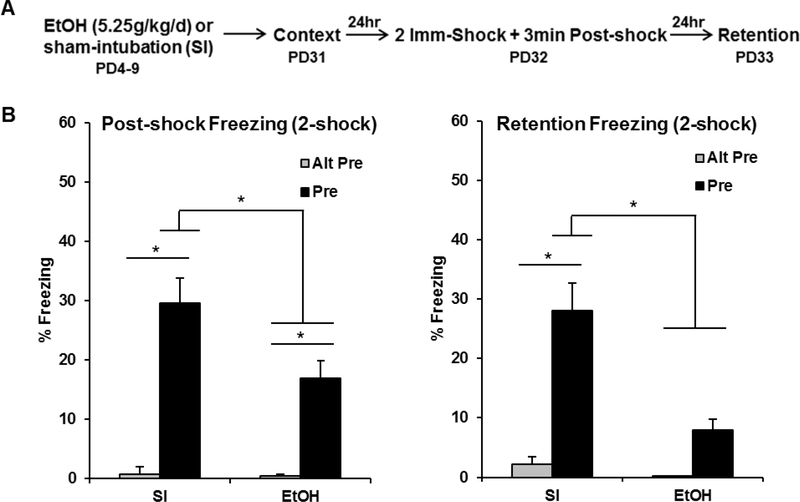
The purpose of Experiment 1B was to determine whether or not increasing the strength of the immediate-shock reinforcement (i.e., by increasing number of shocks to 2 instead of 1) would alter behavioral impairments seen in Experiment 1A. The behavioral design and results for Experiment 1B can be seen in Figure 2. Analyses for Experiment 1B were run on 61 animals distributed across the following groups: EtOH-Alt-Pre (n=9), EtOH-Pre (n=21), SI-Alt-Pre (n=11), and SI-Pre (n=20). Repeated measures ANOVA revealed a significant main effect of Dosing [F(1, 55) = 6.99, p < .01], Exposure [F(1, 55) = 34.04, p < .001], and a significant Dosing × Exposure interaction [F(1, 55) = 4.97, p < .05]. There was no main effect or any interactions involving the repeated measure of Phase (ps > .15). SI-Pre animals froze significantly more than EtOH-Pre animals during the post-shock (p < .05) and retention (p < .001) freezing tests. While there was no difference between EtOH animals and both Alt-Pre groups in retention freezing (p > .30), EtOH animals froze significantly more than the Alt-Pre groups during the post-shock freezing test (p < .05). These results suggest that doubling the amount of shock-reinforcement given during training is not fully effective in rescuing ethanol-induced impairment of the CPFE.
Figure 2.
Behavioral design (A) and mean percent freezing (± SEM) for the 3min post-shock (B) or 5min retention (C) freezing tests. (A) Animals were given alcohol or sham-intubation from PD4–9, and then run through the full three-day CPFE procedure from PD31–33. The US was two immediate shocks. (B) Animals in the SI-Pre group froze significantly higher than the EtOH-Pre group (p < .05), which froze significantly higher than both Alt-Pre groups during the post-shock freezing test (p < .05). (C) The SI-Pre group froze significantly higher than every other group during the retention freezing test (ps < .001), with no difference between the other groups (ps > .20). * indicates p < .05
3.4. Experiment 2: PD4–9 ethanol exposure has no effect on retention test freezing in standard contextual fear conditioning
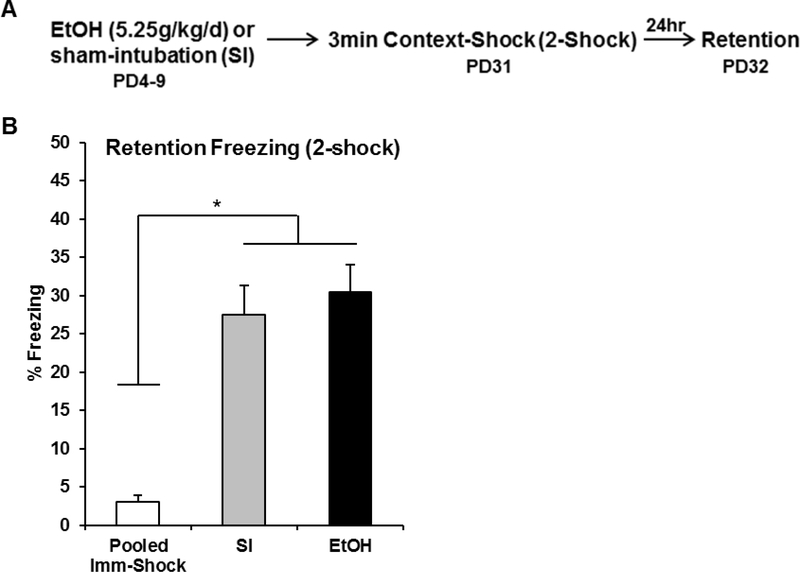
The purpose of Experiment 2 was to examine whether or not ethanol-exposed animals are impaired in standard contextual fear conditioning, in which learning about the context and acquiring a context-shock association occurs within the same trial. The behavioral design and results for Experiment 2 can be seen in Figure 3. Analyses for Experiment 2 were run on 33 animals distributed across the following groups: EtOH-Delayed (n=10), SI-Delayed (n=11), and Pooled-Imm-Shock (n=12; SI=6, EtOH=6). One-way ANOVA revealed a significant main effect of Group [F(1, 30) = 25.47, p < .001]. Both SI-Delayed and EtOH-Delayed groups froze significantly higher than Pooled-Imm-Shock control group (ps < .001), with no difference between the two Delayed groups (p > .50). These results show that ethanol-exposed animals are able to acquire and retain contextual fear when context exposure and foot-shock occur within the same trial.
Figure 3.
Behavioral design (A) and mean percent freezing (± SEM) for the 5min retention (B) freezing test occurring 24hrs after context-shock pairing. (A) Animals were given alcohol or sham-intubation from PD4–9, and then run through the two-day sCFC procedure from PD31–32. The US was two foot-shocks occurring three minutes after chamber entry. (B) There was no difference in retention test freezing between the SI-Delayed and the EtOH-Delayed groups (ps > .58), with both groups freezing significantly higher than an immediate-shock control group collapsed across dosing condition (ps < .001). * indicates p < .05
3.5. Experiment 3: PD4–9 ethanol exposure impairs medial-prefrontal but not dorsal-hippocampal IEG expression during context preexposure
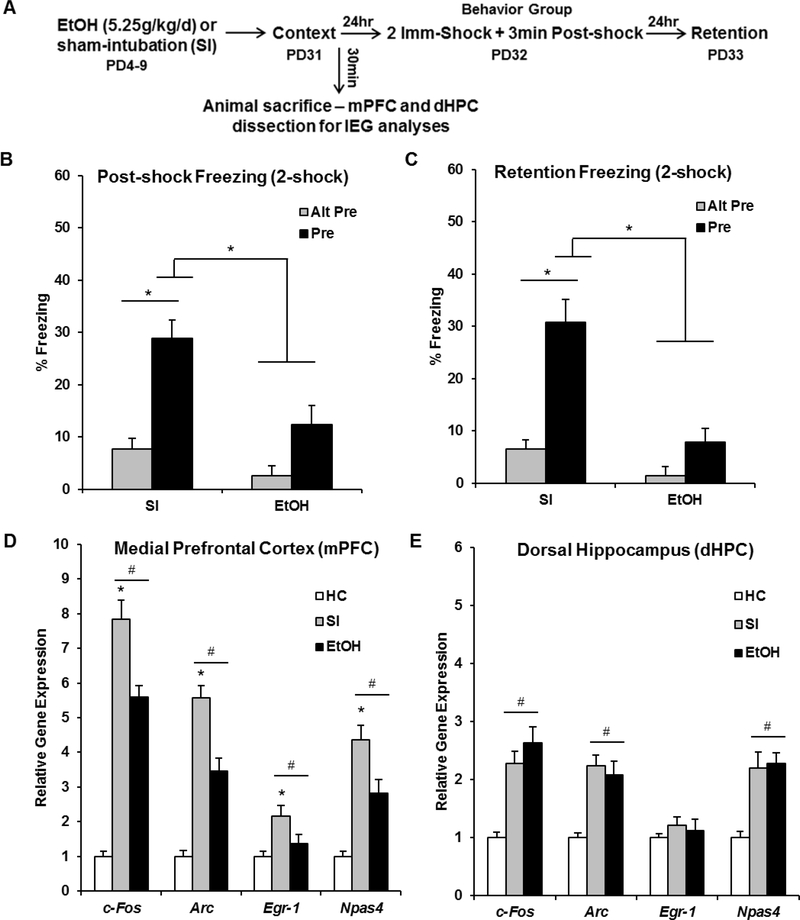
The purpose of Experiment 3 was to determine whether impaired context memory in ethanol-exposed animals is accompanied by disrupted IEG expression in the mPFC and dHPC during context preexposure. The behavioral design and results for Experiment 3 can be seen in Figure 5A-C. Analyses for Experiment 3 were run on 48 animals distributed across the following groups: EtOH-Alt-Pre (n=8), EtOH-Pre (n=13), SI-Alt-Pre (n=11), and SI-Pre (n=16). Repeated measures ANOVA revealed a significant main effect of Dosing [F(1, 42) = 22.15, p < .001], Exposure [F(1, 42) = 34.98, p < .001], and a significant Dosing × Exposure interaction [F(1, 42) = 7.88, p < .01]. There was no main effect or any interactions involving Phase (ps > .40). The SI-Pre group froze significantly more than any other group during both the post-shock and retention freezing tests (ps < .001). Additionally, there was no significant difference between EtOH-Pre and the Alt-Pre control groups (ps > .20).
Figure 5.
Behavioral design (A) and data (B, C), and post-context-preexposure IEG expression in the mPFC (D) and dHPC (E) for the HC, EtOH, and SI experimental groups. (A) Animals were given alcohol or sham-intubation from PD4–9, and then run through the full three-day CPFE procedure from PD31–33. Littermates of this behavior group were sacrificed 30min after context exposure and IEG mRNA expression in the mPFC and dHPC was assayed via qPCR. (B, C) The SI-Pre group froze significantly higher than the EtOH-Pre group and both Alt-Pre control groups during the 3min post-shock and 5min retention freezing tests (ps < .001). (D) The SI group had significantly higher expression of every IEG above both the EtOH and baseline HC control group (ps > .001). The EtOH group had significantly higher c-Fos, Arc, and Npas4 expression than the HC group (ps > .001). (E) Both SI and EtOH groups had significantly higher expression of c-Fos, Arc, and Npas4 above HC control levels, with no difference between the two dosing groups (ps > .18). # indicates significant elevation above the HC group; * indicates a significant difference between SI and EtOH group
Littermates of the behavior group were sacrificed 30 min after context exposure on the preexposure day of the CPFE (see sections 2.6 and 2.7). The IEG results can be seen in Figure 5D-E. Gene expression in Experiment 3 was analyzed using a one-way ANOVA (HC, SI-Pre, and EtOH-Pre) for each gene (c-Fos, Arc, Egr-1, and Npas4) in both the mPFC and dHPC (see Figure 4A and 4B for dissections). Specific F statistics, p values, group n, and outliers removed for all eight one-way ANOVAs for Experiment 3 can be found in Table 2. Post hoc contrasts revealed that, in the mPFC, EtOH animals showed significantly reduced mRNA expression of every IEG (c-Fos, Arc, Egr-1, and Npas4; see Figure 5D) compared to SI animals (ps < .001). However, expression of c-Fos, Arc, and Npas4 in EtOH animals was still significantly above HC control levels (ps > .01). This disruption of IEG expression in the EtOH group was not seen in the dHPC, with both SI and EtOH animals having significantly higher expression of c-Fos, Arc, and Npas4 above HC control levels, with no difference between the two dosing groups (ps > .18; see Figure 5E). These results indicate that neonatal PD4–9 ethanol exposure impairs prefrontal but not hippocampal IEG expression induced by context exposure.
4. Discussion
The current set of experiments examined the disruption caused by neonatal alcohol exposure on context and contextual fear learning in the CPFE in adolescent rats. Consistent with previous CPFE studies [24,27,36,37,57], high binge-like doses of ethanol given over PD4–9 abolished 24-hr retention test freezing (Experiments 1A, 1B, and 3). Importantly, previous research has been unable to elucidate whether this disruption in retention reflects an impairment in preexposure or training day processes. In the current study, ethanol exposure left freezing in sCFC intact (Experiment 2), but post-shock freezing on the training day of the CPFE was abolished in ethanol-exposed animals regardless of reinforcement intensity (i.e., one vs. two shocks) used (Experiments 1A, 1B, and 3). Furthermore, ethanol-exposed animals showed a selective disruption in medial prefrontal but not dorsal hippocampal expression of the IEGs Arc, c-Fos, Egr-1, and Npas4 induced by context preexposure in the CPFE (Experiment 3). Taken together, these results indicate that PD4–9 ethanol exposure disrupts prefrontal but not hippocampal activity- and plasticity-associated gene expression during incidental context learning, which may reflect a disruption in configural memory processes of the CPFE (i.e., acquisition, consolidation, or retrieval of a conjunctive context representation).
Extending previous work examining retention only [24,27,36,37,57], PD4–9 ethanol exposure abolished post-shock and retention test freezing in the CPFE. In contrast, retention freezing in single-trial standard contextual fear conditioning in ethanol-exposed animals was spared. Accordingly, ethanol-induced disruptions in the CPFE cannot be attributed to reduced shock sensitivity, hyperactivity, or impaired context exploration or feature perception. Shortening the interval between context exposure and immediate-shock training to 2hr rescues 24hr retention test freezing in ethanol-exposed animals, suggesting that alcohol exposure does not impair the ability to associate a previously learned context with a shock in the CPFE [57]. Therefore, the observed ethanol-induced deficit in post-shock freezing likely reflects a disruption in the consolidation of the conjunctive context representation after context preexposure in the CPFE. Additionally, because the CPFE requires the mPFC and dHPC during all three phases [43–45,58], whereas single-trial conditioning in sCFC depends on the dHPC but not mPFC [43,59], these results implicate impaired prefrontal mechanisms of the CPFE. This notion is consistent with the observed ethanol-induced disruptions in prefrontal but not hippocampal IEG expression during context learning (Experiment 3).
Our lab has previously characterized disruptions in brain and behavior after different exposure windows in the rat, notably after PD4–6, PD4–9, and PD7–9. Unlike in the PD4–9 or PD7–9 dosing scenarios, PD4–6 ethanol exposure has no effect on retention test freezing in the CPFE [36]. While this might suggest that the disruptive effects of ethanol exposure could be solely attributed to the PD7–9 window, this exposure leaves post-shock freezing on the training day of the CPFE intact [39]. Moreover, impaired contextual fear retention in these animals is associated with reduced prefrontal Egr-1 mRNA expression on the training day of the CPFE [60]. In contrast, we report that the broader PD4–9 exposure results in impaired context memory and prefrontal IEG expression on the preexposure day of the CPFE. Our lab has previously shown that PD4–9 ethanol exposure results in a knockdown of hippocampal c-Fos protein expression and CA1 pyramidal cell loss on the preexposure day [24]. The current study does not replicate this ethanol-induced knockdown in hippocampal c-Fos expression. These different outcomes could reflect procedural differences, i.e., sampling entire dHPC vs. CA1, sampling mRNA vs. protein, one vs. two daily doses, and different amounts of context exposure. Despite this, these results suggest that mechanisms accounting for the more severe behavioral impairment after PD4–9 ethanol exposure likely extend beyond disruptions in hippocampal neuroanatomy and function.
The current results significantly expand upon previous literature demonstrating that developmental ethanol exposure alters prefrontal neuroanatomy and function. Ethanol exposure from PD2–6 or PD4–9 results in decreased dendritic complexity and branching in layer II/III pyramidal neurons [33,61]. Concurrent with reduced dendritic complexity, this exposure also alters voltage-gated Ca2+ channel activity while decreasing dendritic spiking number and duration in layer V pyramidal neurons in the prefrontal cortex [35]. While studies of neonatal exposure are limited, prenatal ethanol exposure alters experience-dependent gene expression in the prefrontal cortex. For example, ethanol exposure throughout gestation results in a decrease in the expression of the IEGs Arc and c-Fos in the prelimbic cortex (PL) in adult rats during wrestling and social interaction behavioral tasks [49,62]. A narrower second-trimester equivalent exposure disrupts the expression of the transcription factors c-Fos and jun-B in the PL and anterior cingulate during testing in a T-maze alternation task [48]. Finally, late gestational exposure also results in decreased c-Fos protein expression in the infralimbic cortex during an open field task in adolescent rats [63]. More research is needed to establish a link between altered prefrontal function and cognitive deficits resulting from neonatal ethanol exposure in rats.
Although the current study failed to find any significant changes in hippocampal gene expression or activity during contextual fear conditioning in ethanol-exposed animals, our findings do not discount previous research demonstrating robust ethanol-induced neuroanatomical and molecular dysfunction in the hippocampus. Ethanol exposure (i.e., via intubation, artificial rearing, or vapor inhalation) during PD2–10, PD4–9, or PD7–9 results in robust decreases in hippocampal CA1 pyramidal cell counts, but CA3 and dentate gyrus neurons are relatively insensitive to treatment [23,25,64]. Sensitivity of CA1 pyramidal neurons is at its peak during this third-trimester equivalent period, with no additional decreases when combined with first and second trimester-equivalent exposure [26]. During this period of cell loss, there is also a stark increase in neuroinflammation, cytokine production (e.g., increased interleukin 1 beta and tumor necrosis factor), DNA methyltransferase activity, and global DNA methylation in the hippocampus in ethanol-exposed rats [28,30,65,66]. Increased DNA methylation generally results in a restrictive state for gene expression and thus could have a negative impact on experience-dependent plasticity [30]. Indeed, PD4–9 ethanol exposure results in altered MAPK/ERK signaling and decreased expression of GluN2B NMDAR subunits, PSD-95, and muscarinic M1 receptors in the hippocampus [28,29,32]. Moreover, both acute and repeated exposure to ethanol abolishes the induction and maintenance of LTP and AMPAR and NMDAR-mediated EPSPs the CA1 ex vivo [31,67]. Ethanol-induced alterations in NMDAR-mediated glutamatergic signaling during development have been linked to apoptotic neurodegeneration across multiple brain regions in the rat [68]. Taken together, these studies demonstrate that the first ten days of life in the rat represents a critical period for ethanol-induced neuroanatomical and molecular insult.
Animal model research has focused on ethanol-induced alterations in hippocampal neuroanatomy and function, and impaired performance in “hippocampal-dependent” behavioral paradigms. For example, ethanol-induced behavioral impairments are seen in spatial navigation in the Morris water maze [25,69– 73], spatial alternation learning in the T-Maze [74,75], delay and trace eyeblink conditioning [56,76–78], in addition to contextual and trace fear conditioning [24,27,80,81,28,29,37–39,51,65,79]. Although deficits are largely attributed to the hippocampus, the medial prefrontal cortex is also required or engaged in the behavioral paradigms in which neonatal alcohol exposure has the most disruptive effects. For example, PD4–9 ethanol exposure severely disrupts trace fear conditioning to a tone CS and to the background context in which the tone is presented [28,29,51,65,79–81]. Successful acquisition and consolidation of a long-term trace fear conditioning memory generally depends on activity and NMDAR plasticity in the medial prefrontal cortex and dorsal hippocampus, and, in some cases, the ventral hippocampus [82–87]. Performance in the Morris Water Maze, another traditionally “hippocampal-dependent” task, is also disrupted by neonatal ethanol exposure in the rat [25,69,71,73]. Manipulations disrupting prefrontal function (e.g., lesions, MAPK/ERK pathway inhibitors) typically interfere with retention but not acquisition of MWM, with the task becoming more prefrontal-sensitive in partial-cue and reversal conditions [88–90]. Interestingly, even rescuing hippocampal CA1 cell loss via Vitamin E supplementation after PD7–9 ethanol exposure does not rescue performance in the MWM in rats, indicating a role for impaired molecular signaling or involvement of other regions [25]. Finally, neonatal ethanol exposure does not impair single-trial sCFC (see Experiment 2; [37]), but abolishes the CPFE across multiple dosing scenarios. Taken together, animal models of FASD have largely focused on examining hippocampal insult, but deficits traditionally attributed solely to the hippocampus could reflect a disruption in extended prefrontal-hippocampal circuitry (e.g., midline thalamus, ventral hippocampus, and amygdala). Therefore, future animal model work should focus more on integrating a systems-level analysis of alcohol insult and underlying neural circuits required for these behaviors.
In summary, our findings demonstrate that PD4–9 ethanol exposure impairs the consolidation of context memory, resulting in abolished post-shock and retention test freezing in the CPFE. This behavioral deficit was associated with a robust impairment in immediate early gene expression in the medial prefrontal cortex of ethanol-exposed animals during the preexposure day of the CPFE. Finally, ethanol-exposed rats were unimpaired during a “prefrontal-independent” but “hippocampal-dependent” standard contextual fear conditioning protocol [43,59], which furthers highlights prefrontal targeting and rules out any “performance effects” of alcohol exposure on behavior. It is important to note that the current findings may be limited to the developmental period of behavioral observation (i.e., in adolescent rats), so more research is needed on the impact of developmental alcohol exposure on behavior across the lifespan. Nevertheless, these findings are important because prefrontal dysfunction is an integral hallmark of FASD in humans, but animal models have thus far largely failed to capture prefrontal dysfunction after third-trimester equivalent exposure. The CPFE has proven to be a promising behavioral paradigm that can facilitate linking alterations in prefrontal and hippocampal function to discrete phases of learning and memory that are impaired by developmental alcohol exposure in animals. More research is needed to establish a link between disrupted brain circuitry and cognitive dysfunction in animal models of FASD.
Highlights.
In the CPFE, developmental alcohol exposure abolishes postshock and retention freezing
Ethanol impairs prefrontal but not hippocampal gene expression during context exposure
Ethanol exposure has no effect on freezing in standard contextual fear conditioning
Evidence for prefrontal cognitive impairment in an animal model of FASD
Acknowledgements
This work is supported by NIH grant R01HD075066–01A1 and F31AA026503–01. We thank both Dr. Jaclyn Schwarz for generously sharing her lab facilities and Lauren Miller for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE, Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies, Dev. Disabil. Res. Rev 15 (2009) 176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- [2].Murawski NJ, Moore EM, Thomas JD, Riley EP, Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies., Alcohol Res. 37 (2015) 97–108. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4476607&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- [3].Rasmussen C, Andrew G, Zwaigenbaum L, Tough S, Neurobehavioural outcomes of children with fetal alcohol spectrum disorders: A Canadian perspective., Paediatr. Child Health 13 (2008) 185–191. [PMC free article] [PubMed] [Google Scholar]
- [4].May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities, JAMA. 319 (2018) 474. doi: 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang Y, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER, Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology, Cereb. Cortex 22 (2012) 1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Wozniak JR, Cortical gyrification is abnormal in children with prenatal alcohol exposure, NeuroImage Clin. 15 (2017) 391–400. doi: 10.1016/j.nicl.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Norman AL, Crocker N, Mattson SN, Riley EP, Neuroimaging and fetal alcohol spectrum disorders, Dev. Disabil. Res. Rev 15 (2009) 209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moore EM, Migliorini R, Infante MA, Riley EP, Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings., Curr. Dev. Disord. Reports 1 (2014) 161–172. doi: 10.1007/s40474-0140020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wozniak JR, Mueller BA, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Sowell ER, The Cifasd, Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol Spectrum disorders (FASD), Brain Imaging Behav. (2016) 1–14. doi: 10.1007/s11682-016-9624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Infante MA, Moore EM, Bischoff-Grethe A, Tapert SF, Mattson SN, Riley EP, Altered functional connectivity during spatial working memory in children with heavy prenatal alcohol exposure, Alcohol. 64 (2017) 11–21. doi: 10.1016/j.alcohol.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ, Alcohol exposure in utero is associated with decreased gray matter volume in neonates, Metab. Brain Dis 31 (2016) 81–91. doi: 10.1007/s11011-015-9771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Willoughby KA, Sheard ED, Nash K, Rovet J, Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood, J Int Neuropsychol Soc. 14 (2008) 1022–1033. doi:S1355617708081368 [pii]r 10.1017/S1355617708081368 [doi]. [DOI] [PubMed] [Google Scholar]
- [13].Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW, Diffusion Tensor Imaging of the Cerebellum and Eyeblink Conditioning in Fetal Alcohol Spectrum Disorder, Alcohol. Clin. Exp. Res 35 (2011) 2174–2183. doi: 10.1111/j.1530-0277.2011.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL, Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome., Neuropsychology. 12 (1998) 146–153. doi: 10.1037/0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- [15].Cheng DT, Jacobson SW, Jacobson JL, Molteno CD, Stanton ME, Desmond JE, Eyeblink classical conditioning in alcoholism and fetal alcohol spectrum disorders, Front. Psychiatry 6 (2015) 1–7. doi: 10.3389/fpsyt.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, Impaired Delay and Trace Eyeblink Conditioning in School-Age Children With Fetal Alcohol Syndrome, Alcohol. Clin. Exp. Res 35 (2011) 250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, Impaired eyeblink conditioning in children with fetal alcohol syndrome, Alcohol. Clin. Exp. Res 32 (2008) 365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- [18].Kodituwakku PW, Neurocognitive profile in children with fetal alcohol spectrum disorders, Dev. Disabil. Res. Rev 15 (2009) 218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Uecker A, Nadel L, Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome, Neuropsychologia. 34 (1996) 209–223. doi: 10.1016/00283932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- [20].Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD, Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task, Behav. Brain Res 143 (2003) 85–94. doi: 10.1016/S0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- [21].Patten AR, Fontaine CJ, Christie BR, A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors., Front. Pediatr 2 (2014) 93. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Driscoll CD, Streissguth AP, Riley EP, Prenatal alcohol exposure: Comparability of effects in humans and animal models, Neurotoxicol. Teratol 12 (1990) 231–237. doi: 10.1016/0892-0362(90)90094-S. [DOI] [PubMed] [Google Scholar]
- [23].Livy DJ, Miller EK, Maier SE, West JR, Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus, Neurotoxicol. Teratol 25 (2003) 447–458. doi: 10.1016/S0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- [24].Murawski NJ, Klintsova AY, Stanton ME, Neonatal alcohol exposure and the hippocampus in developing male rats: Effects on behaviorally induced CA1 c-Fos expression, CA1 pyramidal cell number, and contextual fear conditioning, Neuroscience. 206 (2012) 89–99. doi: 10.1016/j.neuroscience.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marino MD, Aksenov MY, Kelly SJ, Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus, Int. J. Dev. Neurosci 22 (2004) 363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [26].Tran TD, Kelly SJ, Critical periods for ethanol-induced cell loss in the hippocampal formation, Neurotoxicol. Teratol 25 (2003) 519–528. doi: 10.1016/S0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- [27].Hamilton GF, Murawski NJ, St. Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY, Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats, Brain Res. 1412 (2011) 88–101. doi: 10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DuPont CM, Coppola JJ, Kaercher RM, Lindquist DH, Impaired trace fear conditioning and diminished ERK1/2 phosphorylation in the dorsal hippocampus of adult rats administered alcohol as neonates., Behav. Neurosci 128 (2014) 187–98. doi: 10.1037/a0035989. [DOI] [PubMed] [Google Scholar]
- [29].Goodfellow MJ, Abdulla KA, Lindquist DH, Neonatal Ethanol Exposure Impairs Trace Fear Conditioning and Alters NMDA Receptor Subunit Expression in Adult Male and Female Rats, Alcohol. Clin. Exp. Res 40 (2016) 309–318. doi: 10.1111/acer.12958. [DOI] [PubMed] [Google Scholar]
- [30].Otero NKH, Thomas JD, Saski CA, Xia X, Kelly SJ, Choline Supplementation and DNA Methylation in the Hippocampus and Prefrontal Cortex of Rats Exposed to Alcohol During Development, Alcohol. Clin. Exp. Res 36 (2012) 1701–1709. doi: 10.1111/j.15300277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Puglia MP, Valenzuela CF, Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats, Alcohol. 44 (2010) 283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Monk BR, Leslie FM, Thomas JD, The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt, Hippocampus. 22 (2012) 1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hamilton GF, Whitcher LT, Klintsova AY, Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC layer II/III pyramidal neurons, Synapse. 64 (2010) 127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lawrence RC, Otero NKH, Kelly SJ, Selective effects of perinatal ethanol exposure in medial prefrontal cortex and nucleus accumbens, Neurotoxicol. Teratol 34 (2012) 128–135. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Granato A, Palmer LM, De Giorgio A, Tavian D, Larkum ME, Early Exposure to Alcohol Leads to Permanent Impairment of Dendritic Excitability in Neocortical Pyramidal Neurons, J. Neurosci 32 (2012) 1377–1382. doi: 10.1523/JNEUROSCI.5520-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murawski NJ, Stanton ME, Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect, Alcohol. Clin. Exp. Res 35 (2011) 1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murawski NJ, Stanton ME, Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9, Behav. Brain Res 212 (2010) 133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dokovna LB, Jablonski SA, Stanton ME, Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function, Behav. Brain Res 248 (2013) 114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jablonski SA, Stanton ME, Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: Dose-response and post-training consolidation effects, Alcohol. 48 (2014) 35–42. doi: 10.1016/j.alcohol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rudy JW, Context representations, context functions, and the parahippocampal-hippocampal system, Learn. Mem 16 (2009) 573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jablonski SA, Schiffino FL, Stanton ME, Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect, Dev. Psychobiol 54 (2012) 714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rudy JW, O’Reilly RC, Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus., Behav. Neurosci 113 (1999) 867. doi: 10.1037/0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- [43].Heroux NA, Robinson-Drummer PA, Sanders HR, Rosen JB, Stanton ME, Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning, Learn. Mem 3 (2017) 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Robinson-Drummer PA, Heroux NA, Stanton ME, Antagonism of muscarinic acetylcholine receptors in medial prefrontal cortex disrupts the context preexposure facilitation effect, Neurobiol. Learn. Mem 143 (2017) 27–35. doi: 10.1016/j.nlm.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robinson-Drummer PA, Dokovna LB, Heroux NA, Stanton ME, Cholinergic mechanisms of the context preexposure facilitation effect in adolescent rats., Behav. Neurosci 130 (2016) 196–205. doi: 10.1037/bne0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Heroux NA, Osborne BF, Miller LA, Kawan M, Buban KN, Rosen JB, Stanton ME, Differential expression of the immediate early genes c-Fos, Arc, Egr-1, and Npas4 during longterm memory formation in the context preexposure facilitation effect (CPFE), Neurobiol. Learn. Mem 147 (2018) 128–138. doi: 10.1016/j.nlm.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Robinson-Drummer PA, Chakraborty T, Heroux NA, Rosen JB, Stanton ME, Age and experience dependent changes in Egr-1 expression during the ontogeny of the context preexposure facilitation effect (CPFE), Neurobiol. Learn. Mem 150 (2018) 1–12. doi: 10.1016/j.nlm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nagahara AH, Handa RJ, Fetal alcohol exposure alters the induction of immediate early gene mRNA in the rat prefrontal cortex after an alternation task, Alcohol. Clin. Exp. Res 19 (1995) 1389–1397. doi: 10.1111/j.1530-0277.1995.tb00997.x. [DOI] [PubMed] [Google Scholar]
- [49].Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg MJ, Valenzuela CF, Savage DD, Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex, Behav. Brain Res 207 (2010) 290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Heroux NA, Robinson-Drummer PA, Rosen JB, Stanton ME, NMDA receptor antagonism disrupts acquisition and retention of the context preexposure facilitation effect in adolescent rats, Behav. Brain Res 301 (2016) 168–177. doi: 10.1016/j.bbr.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schreiber WB, St. Cyr SA, Jablonski SA, Hunt PS, Klintsova AY, Stanton ME, Effects of exercise and environmental complexity on deficits in trace and contextual fear conditioning produced by neonatal alcohol exposure in rats, Dev. Psychobiol 55 (2013) 483–495. doi: 10.1002/dev.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kelly SJ, Lawrence RC, Intragastric intubation of alcohol during the perinatal period, 2008. doi: 10.1007/978-1-59745-242-7. [DOI] [PMC free article] [PubMed]
- [53].Fanselow MS, Factors governing one-trial contextual conditioning, Anim. Learn. Behav 18 (1990) 264–270. doi: 10.3758/BF03205285. [DOI] [Google Scholar]
- [54].Fanselow MS, Factors governing one-trial contextual conditioning, Anim. Learn. Behav 18 (1990) 264–270. doi: 10.3758/BF03205285. [DOI] [Google Scholar]
- [55].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT Method, Methods. 25 (2001) 402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [56].Brown KL, Goodlett CR, Stanton ME, Neonatal Alcohol Exposure Impairs Acquisition of Eyeblink Conditioned Responses during Discrimination Learning and Reversal in Weanling Rats, Dev. Psychobiol 49 (2007) 243–257. doi: 10.1002/dev. [DOI] [PubMed] [Google Scholar]
- [57].Goodfellow MJ, Lindquist DH, Significant long-term, but not short-term, hippocampal-dependent memory impairment in adult rats exposed to alcohol in early postnatal life, Dev. Psychobiol 56 (2014) 1316–1326. doi: 10.1002/dev.21210. [DOI] [PubMed] [Google Scholar]
- [58].Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW, The Role of the Dorsal Hippocampus in the Acquisation and Retrieval of Context Memory Representations, J. Neurosci 24 (2004) 2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wiltgen BJ, Sanders MJ, Anagnostras SG, Sage JR, Fanselow MS, Context Fear Learning in the Abssence of the Hippocampus, J. Neurosci 26 (2006) 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jablonski SA, Robinson-Drummer PA, Asok A, Rosen JB, Stanton ME, Impairment of the Context Pre-exposure Facilitation Effect in Juvenile Rats by Neonatal Alcohol Exposure is Associated with Decreased Egr-1 mRNA Expression in the Prefrontal Cortex, Behav. Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Granato A, Di Rocco F, Zumbo A, Toesca A, Giannetti S, Organization of cortico-cortical associative projections in rats exposed to ethanol during early postnatal life, Brain Res. Bull 60 (2003) 339–344. doi: 10.1016/S0361-9230(03)00052-2. [DOI] [PubMed] [Google Scholar]
- [62].Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg MJ, Valenzuela CF, Savage DD, Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development, Behav. Brain Res 214 (2010) 66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fabio MC, March SM, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM, Prenatal ethanol exposure increases ethanol intake and reduces C-fos expression in infralimbic cortex of adolescent rats, Pharmacol. Biochem. Behav 103 (2013) 842–852. doi: 10.1016/j.pbb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bonthius DJ, West JR, Alcohol Induced Neuronal Loss in Developing Rats: Increased Brain Damage with Binge Exposure, Alcohol. Clin. Exp. Res 14 (1990) 107–118. doi: 10.1111/j.15300277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- [65].Goodfellow MJ, Shin YJ, Lindquist DH, Mitigation of postnatal ethanol-induced neuroinflammation ameliorates trace fear memory deficits in juvenile rats, Behav. Brain Res 338 (2018) 28–31. doi: 10.1016/j.bbr.2017.09.047. [DOI] [PubMed] [Google Scholar]
- [66].Perkins A, Lehmann C, Lawrence RC, Kelly SJ, Alcohol exposure during development: Impact on the epigenome, Int. J. Dev. Neurosci 31 (2013) 391–397. doi: 10.1016/j.ijdevneu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Puglia MP, Valenzuela CF, Ethanol acutely inhibits ionotropic glutamate receptor-mediated responses and long-term potentiation in the developing CA1 hippocampus, Alcohol. Clin. Exp. Res 34 (2010) 594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ikonomidou C, Price MT, Stefovska V, Ho F, Ethanol-Induced Apoptotic Neurodegeneration and Fetal Alcohol Syndrome, Science (80-. ). 287 (2000) 1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- [69].Goodlett CR, Johnson TB, Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning, Neurotoxicol. Teratol 19 (1997) 435–446. doi: 10.1016/S08920362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- [70].Goodlett CR, Peterson SD, Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats, Neurobiol. Learn. Mem 64 (1995) 265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- [71].Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD, Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats., Behav. Neurosci 121 (2007) 120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- [72].Girard TA, Xing HC, Ward GR, Wainwright PE, Early postnatal ethanol exposure has longterm effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze, Alcohol. Clin. Exp. Res 24 (2000) 300–306. doi: 10.1097/00000374-20000300000007. [DOI] [PubMed] [Google Scholar]
- [73].Johnson TB, Goodlett CR, Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats., Alcohol. Clin. Exp. Res 26 (2002) 83–93. doi: 10.1111/j.1530-0277.2002.tb02435.x. [DOI] [PubMed] [Google Scholar]
- [74].Thomas JD, Wasserman EA, West JR, Goodlett CR, Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: Importance of developmental timing and number of episodes, Dev. Psychobiol 29 (1996) 433–452. doi:. [DOI] [PubMed] [Google Scholar]
- [75].O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH, Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats, Alcohol. 38 (2006) 99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [76].Murawski NJ, Jablonski SA, Brown KL, Stanton ME, Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile rats, Behav. Brain Res 236 (2013) 307–318. doi: 10.1016/j.bbr.2012.08.025. [DOI] [PubMed] [Google Scholar]
- [77].Brown KL, Calizo LH, Stanton ME, Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats, Alcohol. Clin. Exp. Res 32 (2008) 277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- [78].Lindquist DH, Sokoloff G, Milner E, Steinmetz JE, Neonatal ethanol exposure results in dose-dependent impairments in the acquisition and timing of the conditioned eyeblink response and altered cerebellar interpositus nucleus and hippocampal CA1 unit activity in adult rats, Alcohol. 47 (2013) 447–457. doi: 10.1016/j.alcohol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schreiber WB, Hunt PS, Deficits in trace fear conditioning induced by neonatal alcohol persist into adulthood in female rats, Dev. Psychobiol 55 (2013) 352–360. doi: 10.1002/dev.21035. [DOI] [PubMed] [Google Scholar]
- [80].Hunt PS, Jacobson SE, Torok EJ, Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects, Alcohol. 43 (2009) 465–474. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wagner AF, Hunt PS, Impaired trace fear conditioning following neonatal ethanol: reversal by choline., Behav. Neurosci 120 (2006) 482–7. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- [82].Gilmartin MR, Kwapis JL, Helmstetter FJ, Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex, Neurobiol. Learn. Mem 97 (2012) 452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gilmartin MR, Helmstetter FJ, Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex., Learn. Mem 17 (2010) 289–96. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gilmartin MR, Kwapis JL, Helmstetter FJ, NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning., Learn. Mem 20 (2013) 290–4. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Quinn JJ, Oommen SS, Morrison GE, Fanselow MS, Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner, Hippocampus. 12 (2002) 495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- [86].Beeman CL, Bauer PS, Pierson JL, Quinn JJ, Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time., Learn. Mem 20 (2013) 336–43. doi: 10.1101/lm.031161.113. [DOI] [PubMed] [Google Scholar]
- [87].Chowdhury N, Quinn JJ, Fanselow MS, Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice, Behav. Neurosci. 119 (2005) 1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- [88].Sang Jo Y, Hye Park E, Hwan Kim I, Kwon Park S, Kim H, Taek Kim H, Choi J-S, The Medial Prefrontal Cortex Is Involved in Spatial Memory Retrieval under Partial-Cue Conditions, J. Neurosci 27 (2007) 13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Leon WC, Bruno MA, Allard S, Nader K, Cuello AC, Engagement of the PFC in consolidation and recall of recent spatial memory., Learn. Mem 17 (2010) 297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- [90].Fantie BD, Kolb B, An examination of prefrontal lesion size and the effects of cortical grafts on performance of the Morris water task by rats, Psychobiology. 18 (1990) 74–80. doi: 10.3758/BF03327218. [DOI] [Google Scholar]