Abstract
Purpose
In recent years, the relationship between dry eye disease (DED) and psychiatric disorders has been gaining attention. The relationship between dry eye symptoms and psychiatric symptoms has been reported in multiple retrospective studies. However, in previous studies there have been limitations to these observations, such as a lack of close examination of either DED or mood symptoms.
Methods
In this study, we evaluated the psychological state and social functionality of DED patients by administering validated psychiatric tests as well as ophthalmologic examinations twice during the course of DED treatment. Forty subjects (61.3 ± 18.1-years old) received the primary psychiatric assessments and 26 received the secondary psychiatric assessments.
Results
In a cross-sectional examination, we found patients with depressive and/or anxiety symptoms had higher Dry Eye Related Quality of Life Score (DEQ) scores, whereas the objective symptoms of DED did not differ between groups. We also found a positive relationship between depression/anxiety scores and DED subjective symptoms. On the other hand, in longitudinal examination, we found psychiatric symptoms had no impact on subjective and objective DED symptoms throughout the course of DED symptoms.
Conclusions
We found depression and anxiety were related to the subjective symptoms of DED but not the objective symptoms.
Translational Relevance
It is important to pay attention to psychiatric symptoms in patients with DED and an investigation into appropriate treatment strategies for patients with DED in combination with psychiatric symptoms is needed in the future.
Keywords: antidepressant, anxiety, comorbidity, DEQ score, depression
Introduction
Dry eye disease (DED) is a major public health problem in ophthalmology. The prevalence of DED is 5% to 50%.1–3 DED is defined by The Tear Film & Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) as “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.”4 The Asia Dry Eye Society (ADES) defines DED as “a multifactorial disease characterized by unstable tear film causing a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage.”5 DED symptoms include dryness, discomfort, foreign body sensation, pain, itchiness, and so on. The symptoms can last for many years, and the condition has significant negative impacts on patients' lives. DED is a multifactorial disease, which can be caused by a variety of issues, such as collagen disease, Sjögren syndrome, malnutrition, graft versus host disease, medications, and so on. The occurrence rate of dry eye syndrome is markedly increasing in industrialized countries, although the etiology of this condition is multifactorial and largely remains unknown.2
In recent years, the relationship between DED and psychiatric disorders has been gaining attention. It is reported that the prevalence of major depressive disorders is higher in patients with DED.6 Antidepressants are also considered to be a risk factor of DED.7,8 The relationship between dry eye symptoms and psychiatric symptoms has been reported in multiple studies.9–17
However, previous studies had limitations, such as a lack of close examination of either DED or mood symptoms. Some studies did not use internationally validated scales. Moreover, no studies have prospectively followed patients' mood symptoms or DED.
Therefore, we evaluated DED patients' psychological states and social functionality using validated psychiatric scales as well as ophthalmologic examinations during the course of DED treatment. As far as we know, this is the first study that prospectively followed DED patients while focusing on the relationship between mood and severity of DED symptoms.
Material and Methods
Participants
An observational prospective study was conducted between October 3, 2013 and March 31, 2014 at Keio University Hospital, Tokyo, Japan. Inclusion criteria were as follows: (1) patients diagnosed as DED who were followed at the ophthalmology outpatient clinic of the hospital; (2) patients who are over 18-years old; and (3) native speakers of Japanese. Diagnoses were made by experienced ophthalmologists before or at the time of study entry, along with the latest Japanese diagnostic criteria issued in 2006.18 The diagnostic criteria are as follows: (1) presence of dry eye symptoms; (2) presence of qualitative or quantitative disturbance of the tear film (Schirmer test ≤5 mm or break-up time [BUT] ≤5 seconds); and (3) presence of keratoconjunctival epithelial damage (total score of fluorescein staining ≥3 points). Study exclusion criteria were as follows: (1) patients who have complications from anterior ocular segment diseases except for dry eye; (2) patients with a severe posterior ocular disease; (3) patients who had any type of eye operation within the last 3 months; (4) patients with severe physical diseases that can affect eye symptoms; (5) patients who have a severe psychiatric disorder that prevents them from undergoing eye/psychiatric assessment; and (6) patients who are decision-impaired. In this study, healthy controls were not included. As this was a naturalistic observational study each patient's treatment was selected by their physician. During the follow-up, the usual treatment was continued by each physician. The research followed the Declaration of Helsinki and was based on protocol approved by the institutional review board of Keio University Hospital, Japan. Written informed consent was obtained from all participants.
Assessments
We conducted the psychiatric and ophthalmologic assessments described below twice during the study period. The majority of the patients had been seen and followed up with by an ophthalmologist at the clinic before the informed consent. The interval of the follow-up was between 1 and 3 months (median 2 months).
Psychiatric Assessments
Patient interviews were performed by experienced psychiatrists. Patients' sociodemographic characteristics as well as their medical information, such as comorbid diseases, medication history, duration of illness, smoking and alcohol history, occupation, and family history were obtained. If they had a history of psychiatric illness, detailed information regarding onset and treatments was collected. Psychiatric diagnoses were made according to the Diagnostic and Statistical Manual of Mental Disorders-IV using the Mini International Neuropsychiatric Interview (M.I.N.I.).19 Depressive moods and anxiety were evaluated using the Japanese version of the Montgomery-Asberg Depression Scale (MADRS),20 Quick Inventory of Depressive Symptomatology-Japanese version (QIDS-SR16),21 and the Japanese version of the Hamilton Rating Scale for Anxiety (HAM-A).22 Detailed explanations of each assessment scale are shown below.
The Mini-International Neuropsychiatric Interview
In this study, we used the Japanese version of the M.I.N.I. (Kappa coefficient = 0.49–0.93).23 This is a brief structured clinical interview for detecting any psychiatric disorders based on the Diagnostic and Statistical Manual criteria. Its original version was developed by Sheehan and Lecrubier.19
The Montgomery-Asberg Depression Scale
We used the Japanese version of the MADRS (intraclass correlation coefficient = 0.91–1.00).20 This is a 10-item semistructured scale specifically designed to indicate the severity of the subject's depressive condition. The total scores range from 0 to 60, with larger numbers indicating greater severity. Its original version was developed by Montgomery and Asberg.24
Quick Inventory of Depressive Symptomatology
The self-rated, QIDS-SR16 is a self-administered questionnaire that assesses the severity of depressive symptoms over the 7 days prior to assessment.21 The total scores range from 0 to 27, with larger numbers indicating greater severity. In this study, we used the Japanese version of the QIDS-SRJ (Cronbach's α = 0.86).25
Hamilton Rating Scale for Anxiety
In this study, we used the Japanese version of the HAM-A (Cronbach's α = 0.88).22 This is a 14-item semistructured scale to measure the severity of perceived anxiety symptoms. The total scores range from 0 to 56, with larger numbers indicating greater severity. Its original version was developed by Hamilton.26
Ophthalmic Assessments
Subjective symptoms of DED were evaluated using the Dry Eye Related Quality of Life Score (DEQ),27 which consists of 15 questions about diagnostic symptoms of DED and the degree of discomfort, such as difficulty opening eyes, blurred vision, sensitivity to bright light, and problems with reading. All ophthalmic examinations were performed by ophthalmologists with expertise in DED. In order to evaluate the severity of DED, the following three tests were performed: a fluorescein staining test, the tear film BUT with fluorescein, and the Schirmer test. To evaluate the fluorescein staining of the ocular surface, the eye was divided into three equal compartments representing nasal conjunctiva, cornea, and temporal conjunctiva. The maximum staining score for each area was three points, and the maximum total staining score was nine points. For the BUT assessment, the time interval between the last complete blink and the appearance of the first corneal black spot was measured with a stopwatch. The Schirmer test was performed without topical anesthesia. A strip (Whatman No. 41, Showa, Tokyo) was placed at the outer one-third of the temporal lower conjunctival fornix for 5 minutes. The strip was then removed, and the length of wet filter paper was recorded.
Statistical Analysis
The distributions of all variables were inspected using histograms, q-q plots, and the Shapiro-Wilks test before conducting statistical analyses. Participants were divided into two groups regarding the severity of their depressive or anxiety symptoms using a cutoff score for three scales (i.e., ≥12 points for MADRS, ≥14 points for HAM-A, ≥6 points for QIDS). Differences in patient characteristics at baseline between the two groups were examined using X2 analysis for categoric variables and t-test for continuous variables. The relationship between depressive or anxiety symptoms and severity of DED at baseline were examined using Pearson's correlation coefficient. When examining the impact of psychiatric symptoms over the course of DED symptoms, we divided the subjects into two groups based on MADRS for depression or HAM-A for anxiety at baseline and used a mixed model. All statistical analyses were performed using the SPSS software, version 23 (SPSS Inc., IBM, Cary, NC).
Results
Of the 56 subjects who visited the clinic between October 2013 and March 2014, 40 agreed to participate in the study and received the first set of assessments. Fourteen of 40 dropped out from the secondary psychiatric evaluation. The mean age ± standard deviation (SD) of the original 40 subjects was 61.3 ± 18.1, and 30 of 40 (75.0%) of them were female. The mean duration of DED ± SD was 55.38 ± 24.95. Only 2 patients were diagnosed with DED for the first time upon participating in this study. All other patients had already been diagnosed with DED prior to this study. All the patients were treated with one of or a combination of the following: ophthalmic solution (e.g., diquafosol sodium, purified sodium hyaluronate, and rebamipide), eye shampoo, maxacalciol, and/or punctal plugs. Detailed information is shown in Table 1.
Table 1.
Demographic Characteristics of Subjects
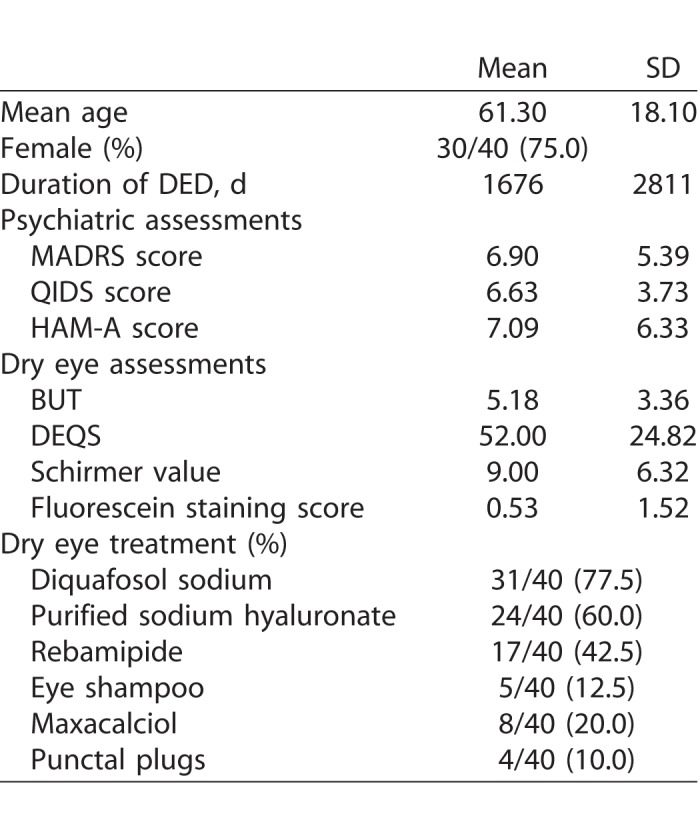
Based on the baseline scores, 6 of 40 (15.0%) of the patients exceeded the threshold of MADRS and 18 of 35 (51.4%) of QIDS for depression. Five of 34 (14.7%) of the subjects exceeded the threshold score of HAM-A for having any anxiety disorder. Two patients had been diagnosed with major depressive disorder and were taking antidepressants during the study period.
Depression, Anxiety Severity, and Dry Eye Symptoms at Baseline
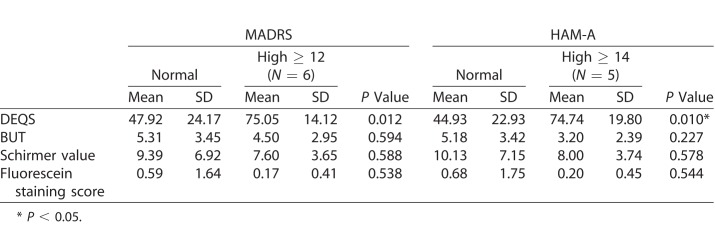
By dividing patients into two groups based on depression severity as determined by their MADRS scores, we found a significant difference between the two groups related to their DEQ scores (i.e., subjective symptoms of DED), whereas there were no statistical differences between the groups regarding other objective measures, such as fluorescein staining test, BUT, and Schirmer score (Table 2). The exact same pattern was found in the results of those patients with anxiety. By dividing patients by HAM-A score we found a significant difference between the two groups regarding DEQ scores but not for objective measures.
Table 2.
The Relationship Between Psychiatric Symptoms and DEQS, BUT, Schirmer Value, and Fluorescein Staining Score at Baseline
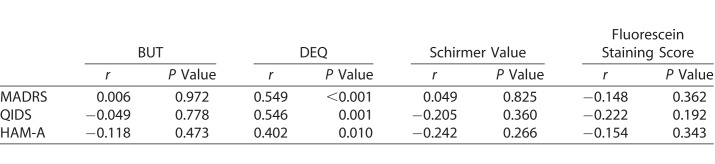
When examining the correlation between the psychiatric and ophthalmologic variables, there was a significant relationship between the MADRS score and DEQ score, but not with objective measures of DED. Similarly, there was a significant relationship between the HAM-A score and DEQ score, but not with objective measures of DED (Table 3).
Table 3.
The Relationship Between Psychiatric Symptoms and Dry Eye Symptoms at Baseline
Dry Eye Symptoms Change Over Time
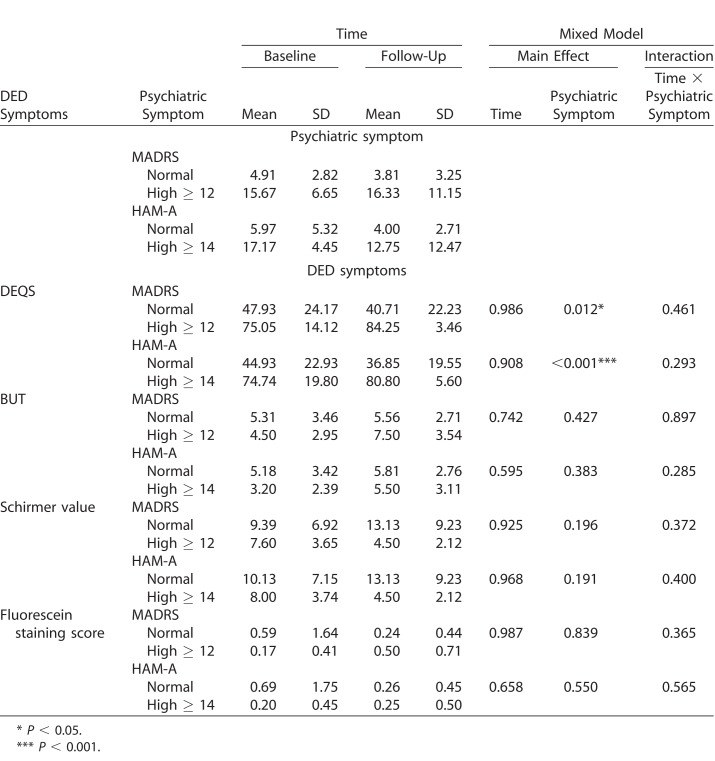
When subjects were divided based on the existence of depressive symptoms, there was no group difference regarding the change of DEQ score over time based on MADRS scores (main effect of time, P = 0.986; main effect of psychiatric symptom, P = 0.012; interaction between time and psychiatric symptom, P = 0.461). Similarly, when the subjects were divided based on the existence of anxiety symptoms, there was no group difference regarding the change of DEQ score over time based on HAM-A scores (main effect of time, P = 0.908; main effect of psychiatric symptom, P = 0.001; interaction between time and psychiatric symptom, P = 0.293). In addition, there were no group differences regarding the change of other objective dry eye scores over time based on MADRS and/or HAM-A scores (Table 4). Removing two participants who were receiving depression treatment did not alter the results. There was a numeric increase in the DEQS score in the high-MADRS group and high-HAM-A group, while there was a numerical decrease in the DEQS score in the normal-MADRS group and normal-HAM-A group. A similar pattern was seen regarding the Schirmer value, in that there was a numeric decrease in the Schirmer value in the high-MADRS group and high-HAM-A group, while there was a numerical increase in the Schirmer value in the normal-MADRS group and normal-HAM-A group. However, none of the differences reached a statistical significance.
Table 4.
DED Symptom Changes Over Time Based on the Existence of Depression and Anxiety Symptoms
Discussion
To the best of our knowledge, this is the first report on longitudinal data focusing on DED and psychiatric symptoms. In cross-sectional examination, we found patients with depressive and/or anxiety symptoms had higher DEQ scores (i.e., had more severe subjective symptoms than patients without depressive or anxiety symptoms), whereas the objective symptoms of DED did not differ between groups. We also found a positive relationship between depression/anxiety scores and DED subjective symptoms. On the other hand, in longitudinal examination, we found psychiatric symptoms had no impact on the course of DED symptoms.
Our cross-sectional findings are mostly in accordance with the previous studies' findings. Li et al.17 reported that the Zung Self Rating Anxiety Scales (SAS) scores were found to be correlated with the Ocular Surface Disease Index (OSDI) and educational level, and the Zung Self Rating Depression Scales (SDS) scores were found to be correlated with OSDI in the DED group. Wan et al.28 found that DED patients are more depressed and anxious than those in the control group, and 29% of DED patients suffer from depression. Hallak et al.13 found DED symptom scores and depression scores evaluated with the Beck Depression Inventory (BDI) were statistically significantly different between DED cases and the control group. Kim et al.29 found depression assessed using the Korean version of the Short Geriatric Depression Scale (SGDS-K) was associated with DED symptoms in elderly subjects with normal or mildly reduced tear production. Mrugacz et al.30 reported that tear levels of interleukin (IL)-6, IL-17, and tumor necrosis factor-α, which had significant correlation with dry eye severity, were higher in depressed patients than in the control group. In our study, too, there was a positive relationship between depression/anxiety scores and DED subjective symptoms. As a side note, in this study it was interesting that the proportion of patients who exceeded the depression threshold was much higher with QIDS, which is a self-report, compared with MADRS, which is assessed by a clinician. This may indicate that patients with DED may be more sensitive to subjective DED symptoms as well as psychiatric symptoms.
In this study, the mean ± SD of DEQ was 52.0 ± 24.8, while Kawashima31 reported that the mean ± SD of DEQ of dry eye patients was 26.8 ± 20.6 in the clinic-based multicenter study. Therefore, some selection bias may have existed.
It would seem natural that patients with stronger depressive or anxiety symptoms feel dry eye symptoms to be more severe. It is reported that the symptoms of a somatic disease can worsen when patients have depression in multiple forms.32,33 This finding shows that it is important for us to consider that it might be beneficial for those who suffer from both DED and psychiatric symptoms, such as depression or anxiety, to shift the focus of their treatment toward their psychiatric symptoms rather than the DED. There is a report that indicates the utilization of antidepressants may be a potential cause of DED.34 However, in our population only two patients were receiving antidepressants, therefore it was difficult to examine whether the antidepressant treatment had any impact on DED. But at the same time, removing two patients who were receiving antidepressant did not change the results.
In this study, depression and/or anxiety symptoms are associated with subjective DED symptoms but not with objective DED symptoms. In Labbé et al.35 the relationship between dry eye symptoms and depression was investigated by targeting patients with DED. It was reported depression score was correlated with subjective dry eye symptoms, but not with the BUT, the Schirmer test, and corneal staining. In addition, Kawashima et al.36 investigated the relationship between DED and subjective happiness among office workers (n = 672) and reported that among subjects who had reported higher level of subjective happiness there was also significantly fewer self-reported dry eye symptoms. They discussed that while subjective DED symptoms besides dryness of the eye, such as ocular fatigue and pain, may also adversely affect quality of life and quality of vision, people with high happiness are less conscious of subjective DED symptoms.36 A similar reasoning may be applied here; in that depressed patients are more concerned about their dry eye symptoms than patients without depressed/anxiety symptoms.
Although we hypothesized that DED prognoses may be different in patients with psychiatric symptoms compared with patients without (i.e., the depressed/anxious group may have worse prognoses compared with psychiatrically stable patients), in actuality, we found the expected pattern both in subjective as well as objective symptoms in our sample. As the change did not reach a statistical significance, we should not draw any conclusions. However, it may indicate some underlying pathophysiology for DED, as described in the next paragraph. If more subjects were included in the study with a longer follow-up period, we might have had more power to find a significant difference. Moreover, patients who participated in this study had been suffering from DED for a long time (mean ± SD, 63.0 ± 99.8) and only two of 40 patients were diagnosed as having DED for the first time during the baseline assessment. Therefore, it is likely that differences in DED prognosis may be hidden due to the ceiling effect of the DED treatment.
Why is DED often found in depressed populations? To date, there is no clear answer to this question; however, some studies indicate a potential connection with the serotonin function that is part of the secretion of tears in the lacrimal gland (LG). Chhadva et al.37 reported that tear serotonin concentration positively correlated with symptoms and signs of dry eye in one cross-sectional study. Imada et al.38 found that the serotonin type 3a receptor expressed in LG acinar cells is involved in tear secretion via intracellular calcium mobilization in mice. In addition, Lambiase39 reported that the concentration of neuropeptide Y (NPY), which has an antianxiolytic effect, was low in the tear fluid of DED patients. We do not know which factor is the cause and which is the effect; however, changes in tear fluid components may be related to depression and anxiety disorders.
Needless to say, not only biological components but also psychological components are likely to contribute to the development of depression/anxiety in DED patients. Ayaki et al.40 reported that dry eye may cause a constant feeling of discomfort or distress that causes mood disorders because dry eye patients may be frequently anxious about their eyes, because symptoms of dry eye are lifelong. Labbé et al.35 discussed that depressive or depressive symptoms in eye patients may be caused by a series of eye symptoms, which have a great impact on patients' daily lives, such as eye discomfort, foreign body sensation, pain, visual impairment, and other symptoms. Yajing et al.41 said that depression in eye patients may also be caused by social factors, doctor visits, and medical expenses.
The findings of the study have to be interpreted in the context of the following limitations. First, the study sample size is small. As we did not have specific information regarding longitudinal data that could be used for sample size calculation, we targeted 50 patients for the study, given the number of patients who visit our dry eye clinic. Although we were able to find significant relationships between psychiatric and subjective symptoms of DED, there may have been type II errors. Moreover, there were many dropouts in the follow up visit (14/40). This limits the interpretation of the results regarding longitudinal change. Second, although we searched for the potential impact of psychiatric symptoms in relation to DED, the follow-up duration may have been too short to detect the difference. Additionally, the assessments were only done twice in the current study. Third, selection bias may have been an issue. In this study, we observed very high DEQ score compared with other study samples. Moreover, patients with depressive and/or anxiety symptoms had severer subjective symptoms than patients without depressive or anxiety symptoms, whereas some previous studies showed the opposite results. Further studies involving more patients are needed. Finally, we were not able to categorize DED based on its etiology, such as Sjögren syndrome and mybome grand disfunction, to examine the interaction with psychiatric status and DED etiology.
Conclusions
In our naturalistic observational study that closely examined dry eye symptoms as well as psychiatric symptoms, we found that depression and anxiety were related to the subjective symptoms of DED but not the objective symptoms. We did not find any correlation between psychiatric symptoms and the prognosis of DED; however, this may be due to the sample selection of this study (i.e., the majority of patients in the study had a long history of DED). A study with a larger sample size and longer duration is warranted. Nevertheless, it is important to pay attention to psychiatric symptoms in patients with DED and an investigation into appropriate treatment strategies for patients with DED in combination with psychiatric symptoms is needed in the future.
Acknowledgments
The authors have no conflict of interest related to the contents of the work.
Disclosure: M. Kitazawa, None; C. Sakamoto, None; M. Yoshimura, None; M. Kawashima, None; S. Inoue, None; M. Mimura, Daiichi Sankyo, Eisai, Pfizer, Shionogi, Takeda, Tanabe Mitsubishi, and Tsumura (F), Daiichi Sankyo, Dainippon-Sumitomo Pharma, Eisai, Eli Lilly, Fuji Film RI Pharma, Janssen Pharmaceutical, Mochida Pharmaceutical, MSD, Nippon Chemipher, Novartis Pharma, Ono Yakuhin, Otsuka Pharmaceutical, Pfizer, Takeda Yakuhin, Tsumura, and Yoshitomi Yakuhin (R); K. Tsubota, Santen Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., AMO Japan KK, Novaliq GmnH, MediProduct Inc., NIDEK Co., Ltd. (C), Jins Co., Ltd, Kowa Comp, Tsubota Laboratory, Inc., and Echo Denki (P); K. Negishi, Fuji Xerox Co., Ltd., Hitachi Automotive Systems, Ltd., Universal View Co., Ltd., Santen Pharmaceutical Co., Ltd., HOYA CORPORATION, Johnson & Johnson Surgical Vision, Inc., Johnson & Johnson K.K., Alcon Japan Ltd., The General Insurance Association of Japan, Kowa Pharmaceutical Co., Ltd., Wakamoto Pharmaceutical Co., Ltd., NIDEK CO., LTD., SANTEC CORPORATION, Carl Zeiss Meditec Co., Ltd., M3 Inc., Kowa Company, Ltd., Tomey Corporation, and B.L.J. Company (F), Santen Pharmaceutical Co., Ltd., Alcon Japan Ltd., Kowa Company, Ltd., Kowa Pharmaceutical Co., Ltd., HOYA CORPORATION, NIDEK CO., AMO Japan KK, SENJU PHARMACEUTICAL CO., LTD., CHUO SANGIO CO., Alcon Pharma K.K, SANTEC CORPORATION, Johnson & Johnson K.K. Carl Zeiss Meditec Co., Ltd., M3 Inc., and Qualitas, Inc. (R); T. Kishimoto, Pfizer Health Research, Takeda, Tanabe-Mitsubishi, Dainippon-Sumitomo, Otsuka, and Mochida (F), Dainippon Sumitomo, Novartis, and Otsuka (C), Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, Novartis, Otsuka, and Pfizer (R)
References
- 1.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss S, Klein R, Klein B. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Craig JP, Nichols KK, Nichols JJ, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Van der Vaart R, Weaver MA, Lefebvre C, Davis RM. The association between dry eye disease and depression and anxiety in a large population-based study. Am J Ophthalmol. 2015;159(3):470–474. doi: 10.1016/j.ajo.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the National United States veterans affairs administrative database. Am J Ophthalmol. 2012;154:340–346.e2. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Wen W, Wu Y, Chen Y, et al. Dry eye disease in patients with depressive and anxiety disorders in Shanghai. Cornea. 2012;31:686–692. doi: 10.1097/ICO.0b013e3182261590. [DOI] [PubMed] [Google Scholar]
- 9.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2003;31:229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee AJ, Lee J, Saw S-M, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86:1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye. 2009;23:688–693. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 12.Liyue H, Chiang PP-C, Sung SC, Tong L. Dry eye-related visual blurring and irritative symptoms and their association with depression and anxiety in eye clinic patients. Curr Eye Res. 2016;41:590–599. doi: 10.3109/02713683.2015.1056804. [DOI] [PubMed] [Google Scholar]
- 13.Hallak JA, Tibrewal S, Jain S. Depressive symptoms in patients with dry eye disease: a case-control study using the beck depression inventory. Cornea. 2015;34:1545–1550. doi: 10.1097/ICO.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na K, Han K, Park Y. Depression, stress, quality of life, and dry eye disease in korean women: a population-based study. Cornea. 2015;34:733–738. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz U, Gokler ME, Unsal A. Dry eye disease and depression-anxiety-stress: a hospital-based case control study in Turkey. Pakistan J Med Sci. 2015;31:626–631. doi: 10.12669/pjms.313.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. 2016;41:1044–1049. doi: 10.3109/02713683.2015.1088955. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36:1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 18.Shimazaki J Definition and diagnosis of dry eye 2006 [in Japanese] Atarashii Ganka [Journal of the Eye] 2007;24:181–184. [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 20.Takahashi N, Tomita K, Higuchi T, Inada T. The inter-rater reliability of the Japanese version of the Montgomery–Asberg depression rating scale (MADRS) using a structured interview guide for MADRS (SIGMA) Hum Psychopharmacol Clin Exp. 2004;19:187–192. doi: 10.1002/hup.576. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 22.Otsubo T, Kudo R, Takashio O, et al. Reliability and validity of the Japanese version of Hamilton Anxiety Rating Scale-Interview Guide. Japanese J Clin Psychopharmacol. 2005;8:1579–1593. [Google Scholar]
- 23.Otsubo T, Tanaka K, Koda R, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59:517–526. doi: 10.1111/j.1440-1819.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery A, Asberg M. A new depression scale designed to be sensitive to change. Brit J Psychiat. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Fujisawa D, Nakagawa ATM. Cross-cultural adaptation of the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-J) Jpn J Stress Sci. 2010;25:43–52. [Google Scholar]
- 26.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the dry eye–related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131:1331–1338. doi: 10.1001/jamaophthalmol.2013.4503. [DOI] [PubMed] [Google Scholar]
- 28.Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. 2016;30:1558–1567. doi: 10.1038/eye.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52:7954–7958. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 30.Mrugacz M, Ostrowska L, Bryl A, Szulc A, Zelazowska-Rutkowska B, Mrugacz G. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. Adv Med Sci. 2017;62:338–344. doi: 10.1016/j.advms.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Kawashima M, Yamada M, Suwaki K, et al. A clinic-based survey of clinical characteristics and practice pattern of dry eye in Japan. Adv Ther. 2017;34:732–743. doi: 10.1007/s12325-017-0487-x. [DOI] [PubMed] [Google Scholar]
- 32.Kang H-J, Kim S-Y, Bae K-Y, et al. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med J. 2015;51:8–18. doi: 10.4068/cmj.2015.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderson SL, Foy R, Glidewell L, McLintock K, House A. How patients understand depression associated with chronic physical disease – a systematic review. BMC Fam Pract. 2012;13:41. doi: 10.1186/1471-2296-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss S, Klein R, Klein B. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 35.Labbé A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97:1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 36.Kawashima M, Uchino M, Yokoi N, et al. Associations between subjective happiness and dry eye disease: a new perspective from the Osaka study. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chhadva P, Lee T, Sarantopoulos CD, et al. Human tear serotonin levels correlate with symptoms and signs of dry eye. Ophthalmology. 2015;122:1675–1680. doi: 10.1016/j.ophtha.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imada T, Nakamura S, Hisamura R, et al. Serotonin hormonally regulates lacrimal gland secretory function via the serotonin type 3a receptor. Sci Rep. 2017;7:6965. doi: 10.1038/s41598-017-06022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129:981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- 40.Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat. 2015;11:889–894. doi: 10.2147/NDT.S81515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Wu X, Lin X, Lin H. The Prevalence of depression and depressive symptoms among eye disease patients: a systematic review and meta-analysis. Sci Rep. 2017;7:46453. doi: 10.1038/srep46453. [DOI] [PMC free article] [PubMed] [Google Scholar]