Figure 4.
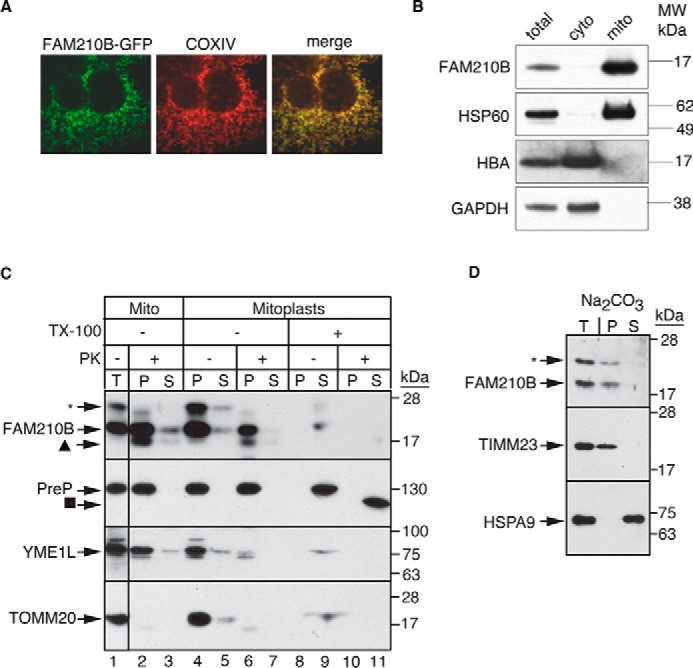
FAM210B localizes to the mitochondrial inner membrane. A, confocal fluorescence microscopy of exogenously expressed FAM210B-GFP (green) in HEK293T cells indicated that FAM210B co-localized with COXIV (red), a mitochondrial resident protein. The overlap is indicated in the merge panel (yellow). B, subcellular fractionation of primary fetal liver cells showed that FAM210B co-sedimented with HSP60 (mitochondrial) but not with HBA and GAPDH (cytoplasmic), indicating its mitochondrial localization. C, isolated mitochondria (Mito, T; lanes 1–3) purified from a MEL cell line stably expressing mouse Fam210b, was treated with proteinase K, which degraded outer membrane proteins (TOMM20) but not intermembrane space proteins (YME1L) or matrix (PreP) proteins (lane 2). Inner mitochondrial proteins, which were impervious to proteinase K treatment, (P) were separated from S proteins by centrifugation. FAM210B co-sedimented with the pellet fraction (lane 2). Mitoplasts (lanes 4–11) were generated by subjecting intact mitochondria to osmotic shock, exposing inner membrane and intermembrane space proteins to proteinase K digestion. Mitoplasts were centrifuged to separate the soluble proteins from the mitoplast pellet. Proteinase K treatment degraded a fraction of FAM210B (lane 6) and completely degraded YME1L but not PreP, suggesting that at least a fraction of FAM210B was situated in the mitochondrial inner membrane or intermembrane space. Triton X-100 (TX-100) treatment liberated FAM210B, PreP, and YME1L from the pellet into the soluble fraction (lane 9). These solubilized proteins were digested by proteinase K, demonstrating specificity of assay (lane 10). The asterisk marks a nonspecific band that was detected with the mouse FAM210B antibody; the triangle marks a likely FAM210B degradation product; and the square marks a core of PreP that is tightly folded and resistant to protease treatment. D, to determine whether FAM210B was an integral inner-membrane protein, isolated mitochondria (T) were analyzed by carbonate extraction. P and S fractions were analyzed by immunoblotting for FAM210B. The blot was probed for TIMM23 as an integral membrane protein control and mortalin as a soluble protein control. An asterisk marks nonspecific bands detected by the FAM210B antibody.
