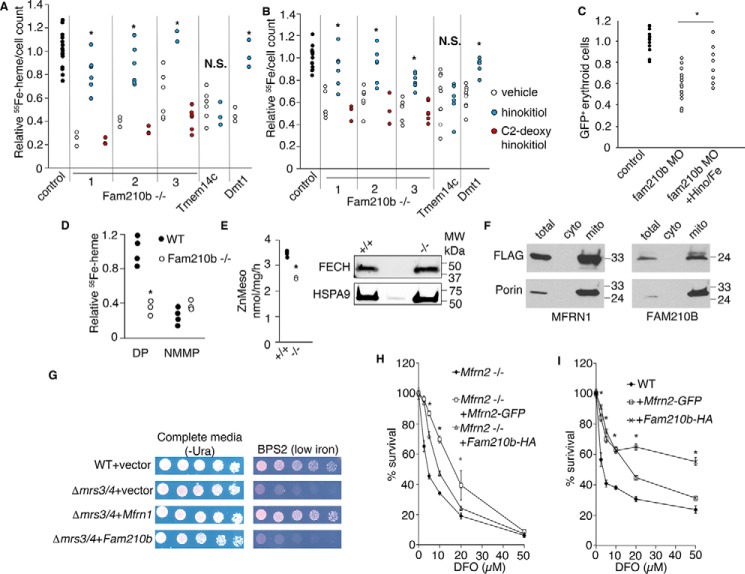
Figure 6.
Fam210B increases the kinetics of mitochondrial iron import in differentiating MEL cells by functioning as an auxiliary factor. A, 55Fe metabolic labeling confirmed a decrease in heme synthesis in differentiating Fam210b (CRISPR1–3), Tmem14c, and Dmt1 deficient MEL cells (black bars). The addition of hinokitiol, a lipid-soluble iron carrier (white bars), restored heme synthesis in Fam210b- and Dmt1-deficient cells, but not Tmem14c-deficient cells. C2-deoxyhinokitiol, which does not chelate iron, did not complement heme synthesis in the Fam210b knockout cells (gray bars) (n = 6). B, 55Fe metabolic labeling confirmed an iron uptake defect in differentiating Fam210b-, Tmem14c-, and Dmt1-deficient cells (black bars). The iron uptake defect in Fam210b- and Dmt1-deficient cells was chemically complemented by hinokitiol (white bars). Hinokitiol did not rescue iron uptake in Tmem14c-deficient cells, which have a porphyrin synthesis defect. C2-deoxyhinokitiol did not rescue the iron deficiency in Fam210b−/− cells (gray bars) (n = 6). C, treatment of fam210b morphant zebrafish embryos (MO) with iron citrate and hinokitiol (MO + Hino/Fe) rescued their anemia (n = 8). D, FECH activity was measured in intact mitochondria isolated from WT and Fam210b−/− MEL cells, using 55Fe and DP as substrates. FECH activity in Fam210b−/− mitochondria was ∼1/3 that of WT controls. Both WT and Fam210b−/− mitochondria had very little measurable FECH activity when NMMP was used as a substrate (n = 4). E, FECH activity was measured in isolated mitochondria from WT and Fam210b−/− MEL cells that were treated with detergent and disrupted by sonication, allowing access of reaction substrates to FECH in the absence of intact membranes. FECH activity was decreased in Fam210b−/− mitochondria, but not to the extent of intact mitochondria (n = 3). F, FLAG-tagged MFRN1 and FAM210B co-localized with porin in Δmrs3/4 yeast, indicating correct mitochondrial localization. G, Mfrn1 expression complemented the growth defect of Δmrs3/4 yeast in low-iron media, whereas Fam210b expression did not. H, survival of Mfrn2−/− fibroblasts in DFO is significantly complemented by expression of Mfrn2-GFP, but less so by expression of Fam210b-HA (n = 3). I, expression of Fam210b-HA and Mfrn2-GFP in WT fibroblasts increase cell survival in the presence of DFO, with Fam210b overexpression possessing a protective effect over a larger dose range than Mfrn2-GFP. Mean ± S.E., n = 3, *, p < 0.05; §, p < 0.1 Student's t test. N.S., not significant.