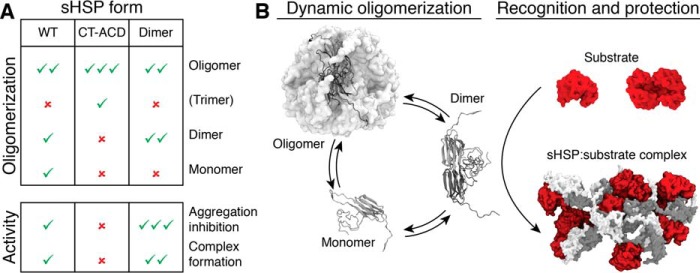
Figure 5.
The sHsp dimer is the major substrate encounter species. A, table summarizing how the disulfide constrained sHsps (CT-ACD and Dimer) alter the population of dodecamer, dimer, or monomer compared with WT and resultant changes in chaperone activity. Check mark indicates presence and abundance of a specific protein form or extent of chaperone activity; × indicates absence of that protein form or activity. B, schematic showing how the dimer, which is the favored form in the dimer mutant and disfavored form in the CT-ACD mutants (as in A) is the major substrate capture form of the sHsp, followed by subsequent assembly of multiple dimers and substrates into heterogeneous complexes.