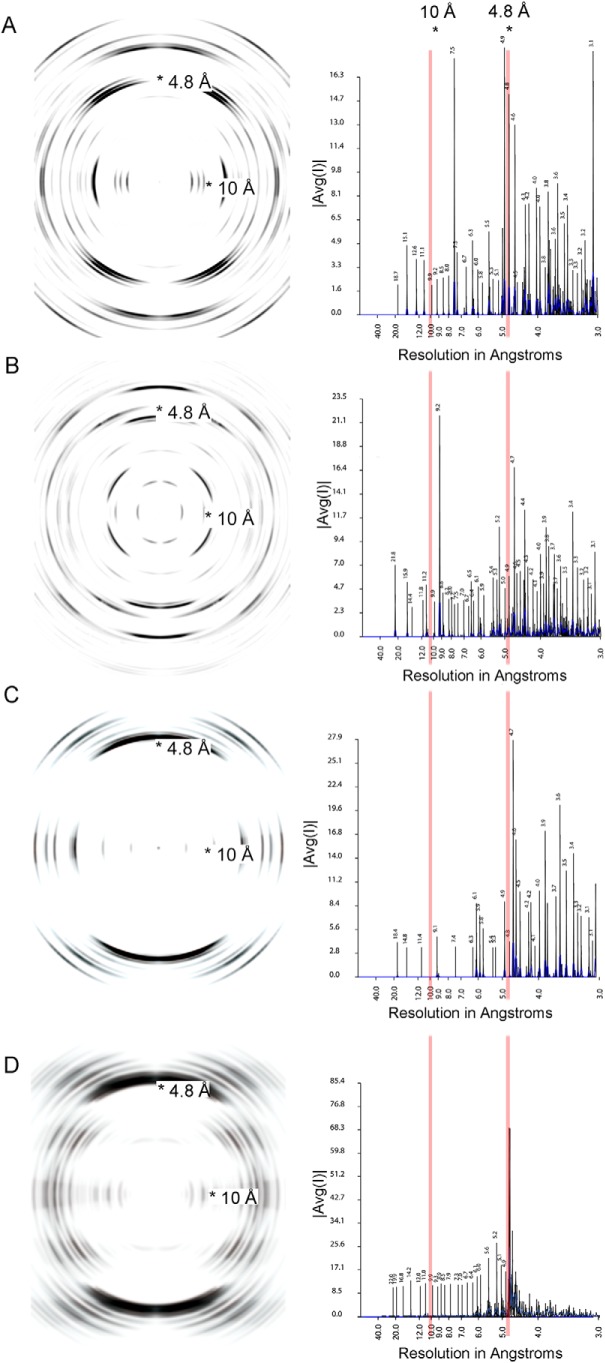
Figure 7.
Fibril diffraction patterns (left column) and their radial integration profiles (right column) for amyloidogenic segments. The fibril diffraction patterns are calculated from the crystal structures of the segments (Fig. 6). The radial integration profiles are calculated by cylindrical averaging of these single crystal diffraction patterns. These patterns show reinforcement of reflections in the vicinity of 4.8 and 10 Å, characteristic of steric zippers. In the right column, |Avg(I)| is the average intensity calculated from a Fourier transform of the atomic structures. A, powder diffraction of ASLTVS. The meridional reflection near 4.8 Å is split due to inclined network of hydrogen bonds of the steric-zipper spine. B, powder diffraction of NFVFGT. C, powder diffraction of YTFGQ. D, cylindrical averaging of single-crystal diffraction data of amyloidogenic segment EFTFTIS from κ AL09. The averaged diffraction data of EFTFTIS show strong reflections near 4.8 and 10 Å, perpendicular to each other. These reflections show the amyloid nature of the structure. The meridional reflection near 9.6 Å indicates that the structure contains an anti-parallel amyloid spine.