Figure 8.
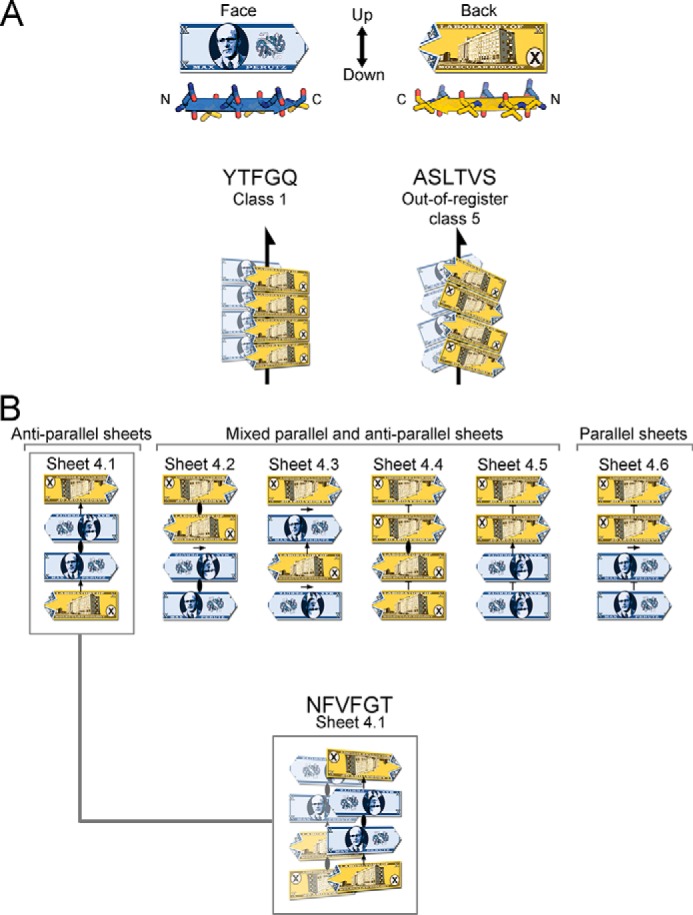
VL steric zipper and sheet geometry of amyloid spines, illustrated schematically with the fictitious Max Perutz banknote. A, Max Perutz banknote represents a protein segment within a steric zipper; it has N and C termini, two distinct faces, and up- and down-direction of hydrogen bonds within a sheet. Arrows represent 21 symmetry axes, meaning that the peptides are related by a 180° rotation about the arrow, and translated one-half of the distance between Max Perutz banknotes along the arrow. The YTFGQ steric zipper belongs to class 1. The ASLTVS steric zipper belongs to class 5. The packing of β-strands is out-of-register with its strands inclined relative to the fibril axis. B, NFVFGT peptide crystallizes in a sheet containing four strands in the asymmetric unit. This sheet belongs to the one of six conceivable arrangements of a sheet with a modulus of four strands and internal symmetry. Arrows represent 2-fold symmetry operators; an ellipse represents a 2-fold symmetry axis perpendicular to the page, and T represents translation.
