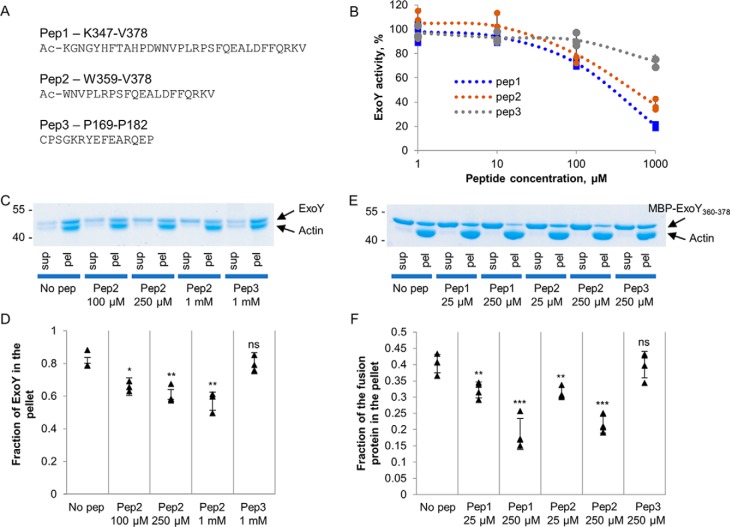
Figure 5.
Inhibition of ExoY activity and actin binding by peptides derived from the C terminus of ExoY. A, sequences of three peptides derived from the C terminus of ExoY used in the study. B, the ExoY guanylyl cyclase activity was measured as described under “Material and methods” in the presence of the indicated concentrations of the different peptides and 1 μm F-actin. ExoY was present at the concentration of 2.2 nm, and 100% of activity corresponds to 35 μmol of cGMP/min/mg. Error bars correspond to standard deviations of three independent experiments. C and D, SDS-PAGE analysis of cosedimentation experiments of 1.5 μm full-length ExoY and 1.5 μm F-actin in the presence of peptides. Fractions of ExoY cosedimented with F-actin in the presence of the peptides were quantified by densitometry using ImageJ software and are presented in D. Error bars correspond to standard deviations of three independent experiments. The difference between the no-peptide (no pep) value and other values was analyzed by the two-tail Student's t test. *, p < 0.05; **, p < 0.01. E and F, SDS-PAGE analysis of cosedimentation experiments with 8 μm fusion protein MBP–ExoY360–378 and 8 μm F-actin in the presence of peptides. The fractions of MBP-fusion360–378 cosedimented with F-actin in the presence of peptides were quantified by densitometry using ImageJ software and are presented in F. Error bars correspond to standard deviations of four independent experiments. The difference between the no-peptide value and other values was analyzed by the two-tail Student's t test. **, p < 0.01; ***, p < 0.001. sup, supernatant; pel, pellet; ns, not significant.