Figure 6.
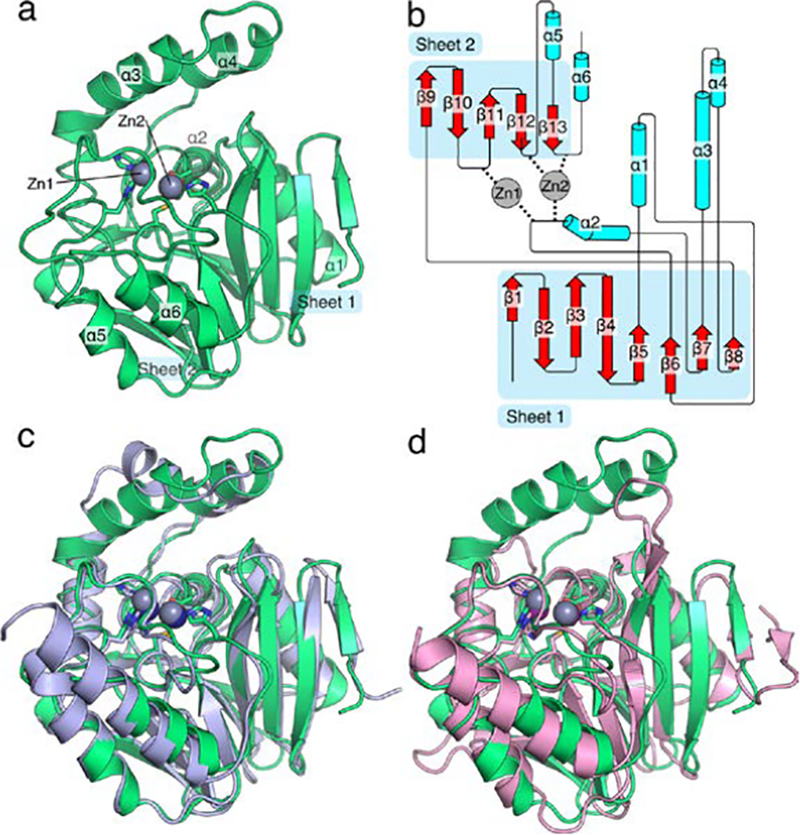
Overall structure of S. smaragdinae SPS-1. The structure of SPS-1 (a) features the canonical αββα metallo-β-lactamase fold with two zinc ions coordinated at the active site. Residues that coordinate zinc ions at the active site are shown as sticks, zinc ions are shown as spheres (grey). A cartoon schematic (b), generated in part by Pro-origami, indicates the organization of the protein and the contribution of loop residues to coordination of Zn1 and Zn2. An overlay of SPS-1 (green) with SPM-1 (blue, PDB ID 4BP0) highlights the similar overall structure (c). An overlay of SPS-1 (green) with NDM-1 (pink, PDB ID 4EXS) highlights a shortened SPS-1 β-hairpin loop between β-strands β3 and β4 and extended α-helices α3 and α4 above the active site for SPS-1 compared to NDM-1 (d). For (c) and (d) residues that coordinate zinc ions at the active site are shown as sticks, zinc ions are shown asspheres (SPS-1: grey, SPM-1: navy blue, NDM-1: purple).
