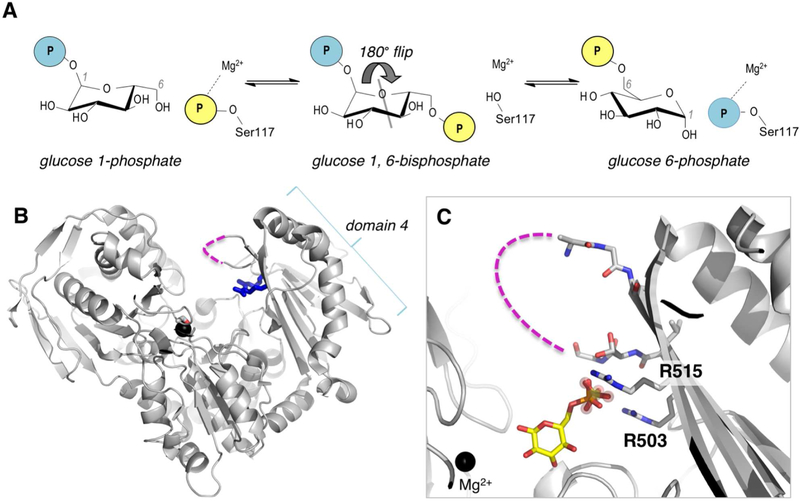
Figure 1. Overview of the mechanism and structure of human PGM1.
(A) A schematic of the catalytic reaction, showing the reversible conversion of glucose 1-phosphate to glucose 6-phosphate. Glucose 1,6-bisphosphate undergoes a 180° reorientation in between the two phosphoryl transfer steps of the reaction (gray line indicates axis of rotation). (B) The crystal structure of WT human PGM1. Ser117, Arg503 and Arg515 are highlighted as sticks; bound metal ion is shown as black sphere. The missing residues in the D4 loop are shown with dashed line. (C) A close-up of the active site of PGM1. The bound sulfate ion in WT enzyme that acts as a structural mimic for the phosphate group of the substrate is shown with spheres; bound glucose 6-phosphate (in yellow, this report) is superimposed. The Mg2+ ion near the site of phosphoryl transfer is shown for reference. Missing residues (507–509) in the D4 loop are shown with dashed line.