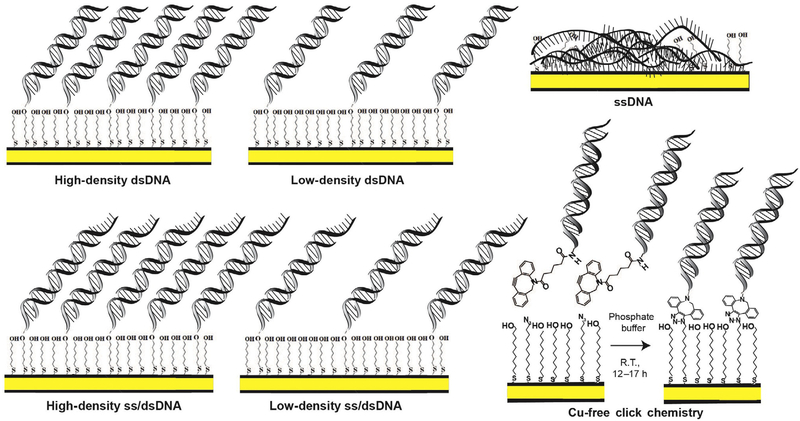
Fig. 3.
Different DNA monolayer morphologies formed on DNA-modified Au electrodes. When duplex DNA is incubated with Mg2+ on an Au surface (yellow), the substrate forms a high-density monolayer of duplex DNA (top left). When incubated on Au in the absence of Mg2+ a low-density duplex DNA monolayer results. DNA containing a single-stranded overhang segment at the interface of DNA monolayer and electrolyte can also be used to form high-density or low-density monolayers for assaying proteins with a preferred primed end substrate (bottom left). When single-stranded DNA is incubated on the Au electrode, the substrate adheres to the surface and passivates the Au, precluding observation of a redox signal (top right). Finally, Cu-free click chemistry can be used to form a DNA monolayer on an Au electrode surface (bottom right). Azide-terminated alkanethiol-modified Au electrode is incubated in 1:1 mix of mercaptoundecanol and 1-azidoundecane-11-thiol in ethanol for about 4h. 50 μM DBCO-modified dsDNA in DNA phosphate buffer is incubated with modified Au electrodes for 12–17h to let the cyclooctyne-based copper-free click reaction proceed. DBCO-modified DNA clicks only to the azide terminal groups, so that the binding density depends on the initial azide content. These monolayers all serve as useful conditions or controls when characterizing redox activity of a DNA-binding enzyme.