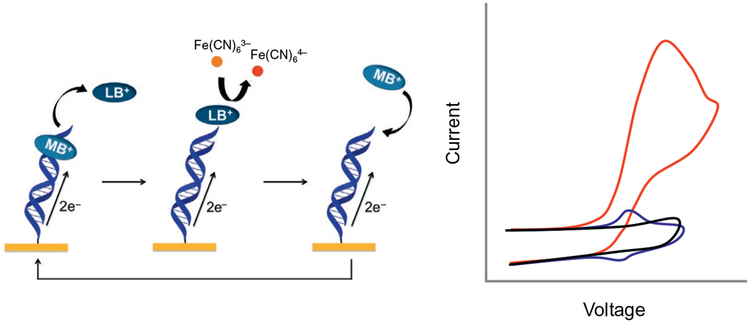
Fig. 5.
Electrocatalytic cycle between free methylene blue (MB) and ferricyanide on a DNA-modified electrode. MB in its oxidized form is intercalated into the DNA base stack. Upon reduction of MB to leucomethylene blue (LB) via DNA-mediated CT, the affinity of the LB for DNA is lowered, and LB is no longer intercalated. The reduced LB is capable of reducing ferricyanide that is freely diffusing in solution. The LB is then reoxidized to MB and can reintercalate into the DNA. The ferricyanide acts as a diffusing electron sink in solution for the redox probe MB. Electrostatic repulsion prevents ferricyanide from penetrating the negatively charged DNA film. A cyclic voltammetry at a DNA-modified electrode of ferricyanide (black), MB (blue), and ferricyanide and MB (red).