Figure 4.
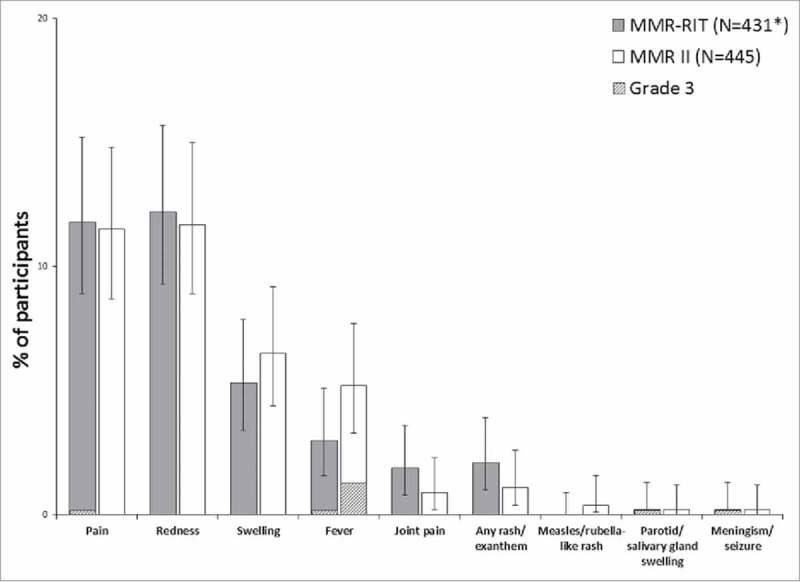
Incidence of solicited injection site (Day 0–3) and general adverse events (Day 0–42) (total vaccinated cohort). Footnote: N, number of participants with the documented dose with local symptoms sheets completed *Except for pain, redness, and swelling, for which MMR-RIT (N = 433). Fever: temperature ≥38°C. Grade 3 was defined as: limb was painful at rest, which prevented normal everyday activities (pain); diameter >50 mm (redness and swelling); temperature >39.5°C (fever); adverse event preventing normal, everyday activities (joint pain, rash/exanthem, meningism/seizure); swelling with accompanying general symptoms (parotid/salivary gland swelling). The error bars represent the upper and lower limits of the two-sided 95% confidence intervals obtained using the Clopper Pearson method.
