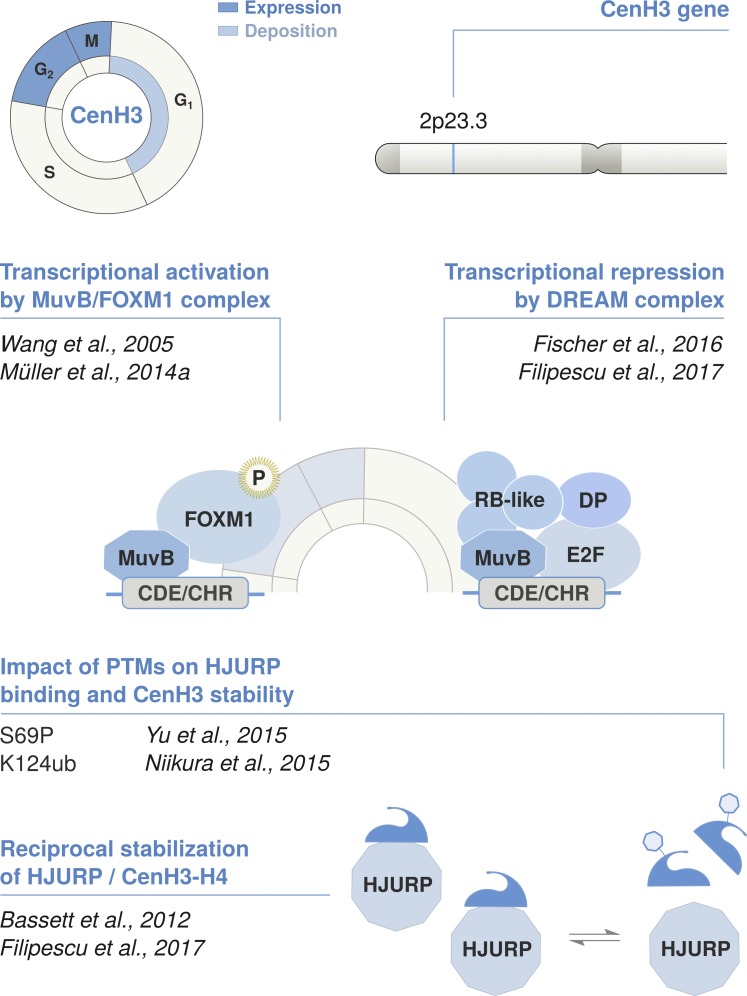
Figure 4.
Cell cycle timing and regulation of the centromeric variant CenH3. The non-replicative variant CenH3CENP-A is deposited at centromeres by its dedicated chaperone HJURP in telophase/early G1. Its expression is regulated in a cell cycle–dependent manner and peaks in G2/M. CenH3CENP-A is encoded by a single multi-exon gene located outside of the histone clusters that undergoes conventional pre-mRNA processing via splicing and polyadenylation. Transcription is coordinated in concert with other late cell cycle genes, including HJURP. This is regulated in cis by the CHR/CDE motif in their promoter region. The recruitment of the DREAM complex at the CHR is thought to promote transcriptional repression during G1. At the beginning of S phase, the MuvB core of the DREAM complex remains bound to the CHR/CDE while other components dissociate and are replaced by B-MYB. The B-MYB-MuvB (MMB) complex recruits FOXM1 in late S phase. B-MYB is degraded upon phosphorylation, whereas the progressive phosphorylation of FOXM1 leads to its activation in G2/M. Both CenH3 and HJURP are induced at the same time and mutually stabilize each other at the protein level. The reciprocal stabilization is affected by posttranslational modifications on the N-terminal tail of CenH3, which further regulate the timing of deposition. S69 phosphorylation prevents interaction with HJURP and premature loading, while K124 ubiquitylation favors HJURP binding and might contribute to their stabilization.