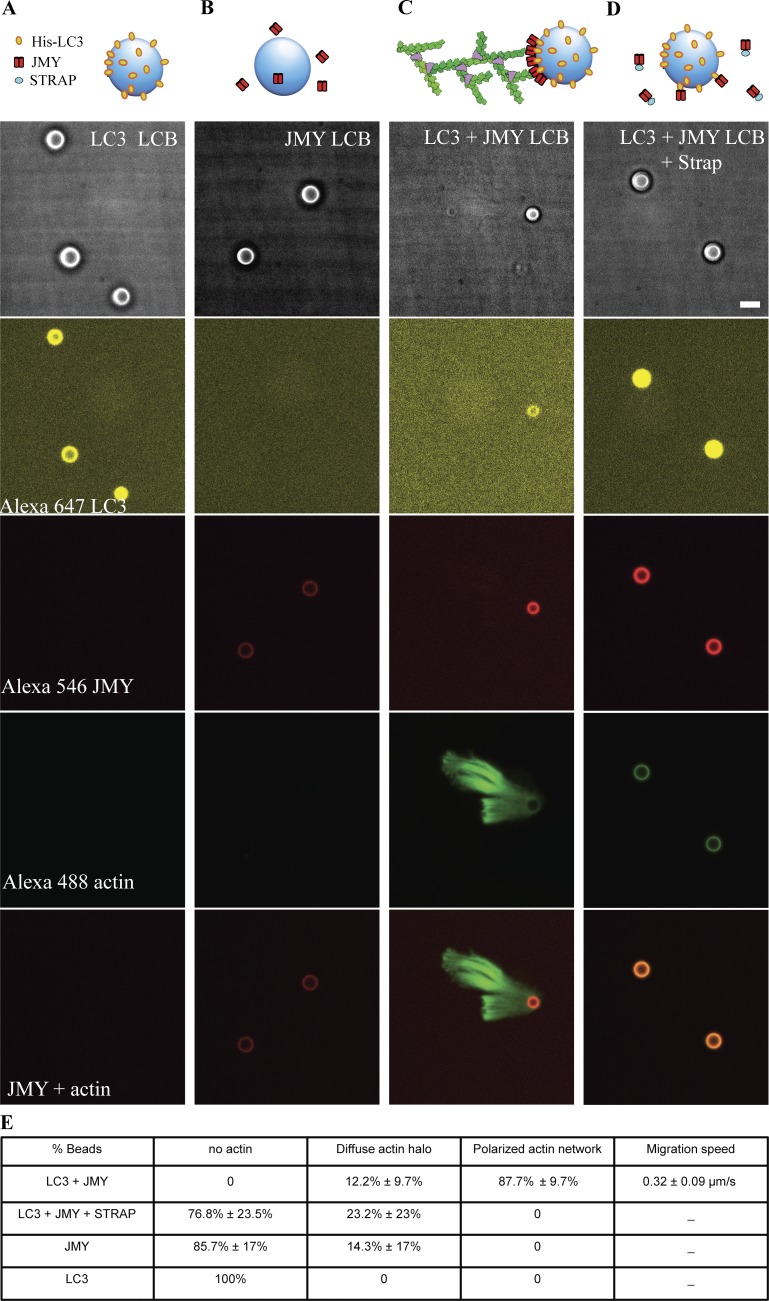
Figure 8.
Reconstitution of LC3- and JMY-dependent actin comet tail formation from purified components. We mixed lipid-coated 4.5-µm glass microspheres with his-tagged LC3 (A); full-length JMY (B); both his-tagged LC3 and JMY (C); and STRAP together with his-tagged LC3 and JMY (D). From top to bottom: bright-field images; Alexa Fluor 647–labeled LC3; Alexa Fluor 546–labeled JMY; Alexa Fluor 488–labeled actin; merger of fluorescent signal from Alexa Fluor 546–labeled JMY and Alexa Fluor 488–labeled actin. (E) Quantification of actin network on lipid-coated bead surface. Buffer conditions: 8 µM actin (10% labeled with Alexa Fluor 488), 200 nM Arp2/3, 400 nM Capping protein, 8 µM profilin, 2 µM STRAP as noted, 1 mg/ml BSA, 1 mg/ml β-casein, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, and 20 mM Hepes, pH 7.0. Temperature: 23°C. Scale bars, 5 µm.