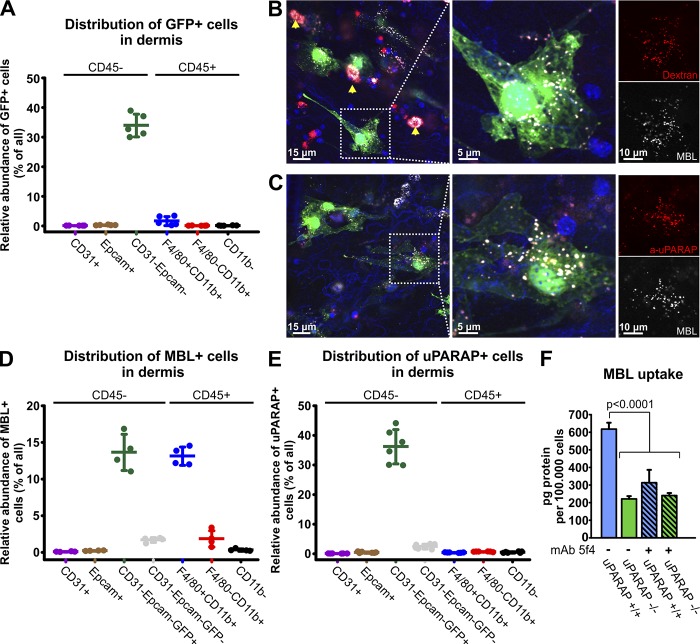
Figure 3.
Flow cytometry and confocal imaging reveals in vivo uptake of the collectin MBL by uPARAP-positive fibroblasts in dermis. (A) Flow cytometry–based quantification of GFP-positive cells in dermal cell populations acquired from col1a1.GFP transgenic mice. (B and C) Uptake of Alexa Fluor 647–labeled murine MBL-A (white) by dermal fibroblasts in vivo was assessed using confocal imaging 24 h after subcutaneous injection into col1a1.GFP mice. MBL was coinjected with either Alexa Fluor 546–labeled 10 kD dextran (B, red) or Alexa Fluor 555–labeled anti-uPARAP mAb 2h9 (C, red). Fibroblasts (green) with a vesicular uptake of MBL and dextran (B) as well as MBL and the anti-uPARAP antibody (C) were abundant. A nonfibroblast cell population with MBL uptake was also observed, and a very potent uptake of dextran in these cells positively identified them as dermal macrophages (arrows). Hoechst 33342 was used for visualization of cell nuclei (blue). (D and E) Quantification of the number of MBL-associating (D) and uPARAP-positive cells (E) within dermal cell populations assessed using flow cytometry 24 h after subcutaneous injection of Alexa Fluor 647–labeled MBL or Alexa Fluor 647–labeled anti-uPARAP mAb 2h9 into col1a1.GFP mice. Injection of Alexa Fluor 647–labeled irrelevant murine IgG was used as a negative control (E). n = 5 (A), 4 (D), and 6 (E). (F) Internalization of radiolabeled MBL in cultured primary dermal fibroblasts from uPARAP−/− mice and WT (uPARAP+/+) littermates. Internalization was examined in the absence or presence of the uPARAP function-blocking antibody mAb 5f4. One-way ANOVA was used to test for statistical significance. Analysis was performed in triplicate. Data are presented as mean ± SD.