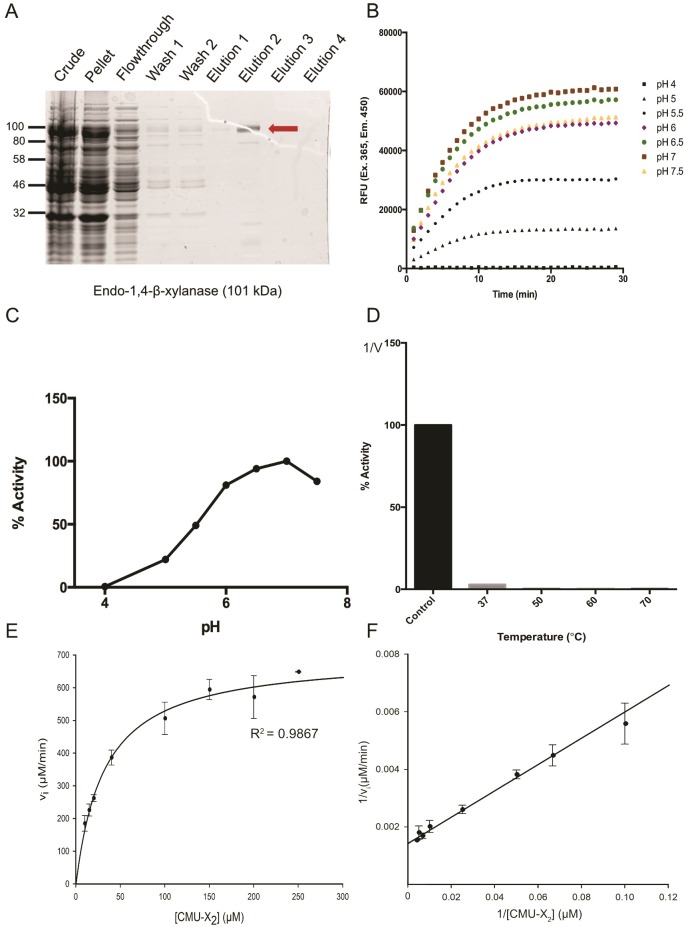
Fig 6. Characterization of endo-1,4- β-xylanase.
A) Putative endo-1,4-β-xylanase was purified by 6xHis purification. B) 0.6 μg of endo-1,4-β-xylanase was combined with 100 μM of CMU-X2 to assess enzyme activity at different pH values. Samples were assessed via fluorescence in sodium citrate buffer (pH 4, 5, 5.5, 6) or sodium phosphate buffer (pH 6.5, 7, 7.5). A reading of raw fluorescent units (450 nm) was taken every minute for 30 minutes at 37°C. C) Relative activity of endo-1,4-β-xylanase at different pH levels compared to pH 7. D) Thermostability of endo-1,4-β-xylanase was assessed. Samples containing 0.6 μg of endo-1,4-β-xylanase in 50 mM sodium phosphate buffer were incubated for 30 minutes at 37°C, 50°C, 60°C, or 70°C before addition of 100 μM of CMU-X2 and fluorescence detection. Control is unincubated endo-1,4-β-xylanase. E) Michaelis-Menten plot was generated with initial reaction rate against substrate concentrations above at 37°C, pH 7. F) Lineweaver-Burk plot was used to generate kinetic constants KM, Vmax, and kcat.