Abstract
Rationale:
Autoimmune hepatitis (AIH) is characterised by interface hepatitis. However, some acute cases exhibit atypical centrilobular necrosis with mild portal inflammation. Detailed histological and ultrastructural analyses of acute AIH are limited.
Patient concerns:
A 44-year-old female was admitted to our hospital with jaundice, general fatigue and liver dysfunction. Her transaminase levels were elevated, her immunoglobulin G level was 1735 mg/dL and her anti-nuclear titres were ×80.
Diagnosis:
AIH was diagnosed, and histochemical examination of a liver biopsy showed the presence of atypical histological features of prominent centrilobular necrosis and central vein and hepatic sinusoidal endotheliitis. Electron microscopy showed that dendritic cells (DCs) and lymphocytes were attached to disrupted liver sinusoidal endothelial cells (LSECs) in hepatic sinusoids and that DCs attached to LSECs via pseudopods in the central vein.
Interventions and outcomes:
The patient was started on 40 mg/day prednisolone to control the hepatic inflammation. Her aspartate and alanine aminotransferase levels started declining after prednisolone was initiated. Three weeks later, these levels had normalised. The dosage of prednisolone was gradually decreased as liver function improved. The patient remains under observation and continues to receive 2.5 mg prednisolone.
Lessons:
An important marker of acute AIH may be the presence of activated DCs in the hepatic sinusoids and central vein.
Keywords: dendritic cells, hepatitis, sinusoidal vein
1. Introduction
Autoimmune hepatitis (AIH) is a form of hepatitis that is characterised by autoantibodies and hypergammaglobulinaemia and, at the histological level, by chronic active hepatitis that presents as portal inflammation with fibrosis, interface hepatitis, the formation of rosettes by hepatocytes and prominent lymphocytic/plasmocytic infiltration.[1]
Histologically, centrilobular necrosis is defined as a lesion that
-
1)
affects the centrilobular area of the liver,
-
2)
is associated with mononuclear cell inflammation around the central venous branch and
-
3)
is accompanied by varying degrees of zonal hepatocyte dropout.[2]
A previous study reported 41 cases of acute AIH, of which 13 exhibited centrilobular necrosis.[3] Another study reported that central vein endotheliitis is an uncommon finding; indeed, it was present in only 6 of 51 biopsy samples taken from liver transplant patients with de novo acute AIH.[4]
Sinusoidal endotheliitis is associated with active hepatic inflammation in liver allografts and can serve as a predictive marker of liver allograft rejection.[5]
Multiple lines of evidence show that dendritic cells (DCs), which are professional antigen-presenting cells, participate in the onset of autoimmune diseases.[6] For example, DCs take up necrotic cell-derived self-antigens and cross-present them to CD8+ T cells.[7]
Of particular importance for the present case report, it has also been observed that endothelial cells may aid the transmigration of plasmacytoid DCs from blood vessels into the site of immune action (eg, the site of infection) via interactions between intercellular adhesion molecule-1 (ICAM-1) on endothelial cells and lymphocyte function adhesion molecule (LFA) on the plasmacytoid DCs.[8]
The acute presentation of AIH has not been studied to date. Moreover, immunohistochemical and ultrastructural changes in the central vein in AIH have not been reported. Here, we report a patient with acute AIH. The immunohistochemical and electron microscopic analyses of a liver biopsy obtained 4 days after presentation are described here.
2. Case report
A 44-year-old female was admitted to our hospital with jaundice, general fatigue and liver dysfunction. Her medical history revealed that she had no health issues before her admission to the hospital. Specifically, the patient reported no evidence of liver dysfunction during regular medical check-up evaluations. She also did not have a history of hepatobiliary disease. She denied being exposed to hepatotoxic drugs or chemicals or alcohol. Furthermore, there was no parenteral exposure or history of transfusion with blood products.
The blood test results were as follows: total bilirubin, 8.1 mg/dL; direct bilirubin, 5.8 mg/dL; aspartate aminotransferase, 1246 IU/L; alanine aminotransferase, 631 IU/L; alkaline phosphatase, 476 IU/L; and γ-glutamyl transpeptidase, 390 IU/L. Serological analyses for viral causes of hepatitis, including the hepatitis A, B and C viruses, the herpes simplex virus and the Epstein Barr virus, were negative. Other relevant data included the following: immunoglobulin (Ig) G, 1735 mg/dL; IgM, 67 mg/dL; anti-nuclear antibody, ×80 (cut-off, <×40); anti-smooth muscle antibody, ×20 (cut-off, <×20); liver-kidney microsomal autoantibody type 1, negative (cut-off, <5.0); and mitochondrial M2 antibody, negative (cut-off, <5.0). On computed tomography, there were no signs of a mass lesion. The patient was referred to our hospital 2 days after being admitted to a local clinic. A needle liver biopsy specimen obtained 4 days after admission was subjected to histological examination (Fig. 1). Low-magnification images of the hematoxylin and eosin-stained tissue (Fig. 1a) showed that while the overall acinar architecture was preserved, all centrilobular regions exhibited perivenular hepatocyte necrosis that was sharply demarcated by a distinct rim of necro-inflammatory activity that featured extensive erythrocytic and dense lymphocytic infiltration. In addition, the liver biopsy showed only mild inflammation in the portal tract (P in Fig. 1a) that consisted largely of lymphocytes; plasmacytosis was not observed. The central vein (c in Fig. 1a) was infiltrated with subendothelial lymphocytic cells (Fig. 1a). This was also observed in high-magnification images of the central vein (arrow in Fig. 1b). Similarly, high-magnification images of the hepatic sinusoids (S in Fig. 1a) revealed infiltration of the sinusoids with subendothelial lymphocytes (black arrowheads) and endothelial lifting and damage (Fig. 1c). Veno-occlusive changes were not noted. Human leukocyte antigen typing showed that the patient was HLA-DRB1∗0405 (DR4)-positive. The diagnosis at that point was probable AIH.1 The interactions between mononuclear cells, vascular endothelial cells and sinusoidal endothelial cells noted on histochemistry led us to measure serum levels of soluble (s)ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1). The data revealed that sICAM-1 (3620 ng/mL; normal, 82.5–276) and sVCAM-1 (2790 ng/mL; normal, 362–892) levels were elevated markedly. Next, we performed immunohistochemical analysis of serial sections to examine whether the vascular endothelium expressed molecules associated with cell migration. ICAM-1 expression was upregulated in sinusoidal and hepatic vein endothelial cells (Fig. 2a). LFA-1 immunoreactivity was detected in lymphocytes around the central and portal veins (Fig. 2b). Vascular cell adhesion molecule-1 (VCAM-1) expression was observed in hepatic sinusoids and the central vein (Fig. 2c). Next, we investigated the cellular architecture of the biopsied tissue by electron microscopy. Ultrastructure images of the hepatic sinusoids (Fig. 3a and b) revealed the presence of spindle-shaped, ballooning liver sinusoidal endothelial cells (LSECs) with DC and lymphocytic infiltration in the space of Disse. The adjacent liver tissue exhibited coagulation necrosis (white arrow in Fig. 3a). High-magnification images of the hepatic sinusoids (S in Fig. 3a) showed that the DCs were attached to the LSECs and lymphocytes via pseudopods (Fig. 3b). Similarly, ultrastructure images of the central vein space (Fig. 3c–e) showed detachment of the endothelial lining cells with DC and lymphocytic infiltration (Fig. 3c). Numerous inflammatory cells, including lymphocytes, DCs and macrophages, adhered to the vascular endothelial cells in the central vein (Fig. 3c–e). The DCs and lymphocytes attached to the endothelial cells of the central vein via pseudopods (Fig. 3d and e).
Figure 1.
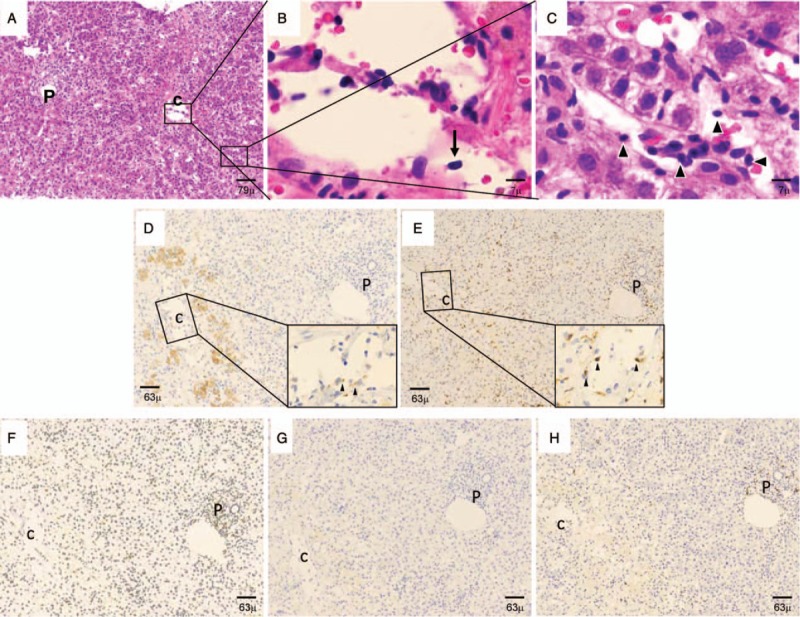
Light microscopic findings of the needle liver biopsy specimen obtained 4 days after admission. (A–C) Haematoxylin and eosin stain. (A) Low-magnification image. This image indicates the presence of lobular hepatitis and central zonal necrosis, which are strongly suggestive of autoimmune hepatitis. (B and C) High-power images of (A). (B) A closer view of the central zonal necrosis. The central vein is infiltrated with subendothelial lymphocytes (black arrow). (C) A closer view of the sinusoidal endotheliitis. The sinusoidal space has widened and a subendothelial lymphocytic infiltrate (black arrowheads) can be observed in the hepatic sinusoids along with endothelial lifting and damage. (D–H) Immunohistochemical expression of CD83, CD8, CD4, CD56 and CD20. (D) CD83+ DCs. Several DCs that expressed CD83 in their cytoplasm were detected in clusters in the hepatic sinusoids and around the central vein. These cells were attached to the endothelium of the central vein (arrowheads in the high-magnification inset image of the central vein). (E) CD8+ T cells. There were many T cells that expressed CD8. They had a branch-like morphology in the hepatic sinusoids and central vein. Some CD8+ T cells also infiltrated the portal tract. The CD8+ T cells in the central vein were attached to the central vein endothelium (arrowheads in the high-magnification inset image of the central vein). (F) CD4+ T helper cells. Helper T cells that expressed CD4 were mainly present as infiltrates in the portal tract. However, a few were also noted in the central vein and hepatic sinusoids. (G) CD56+ natural killer cells. A few CD56+ natural killer cells were found in the central vein and hepatic sinusoids. (F) A few CD20+ B cells infiltrated the portal tract. Fewer such cells were detected in the central vein and hepatic sinusoids. P, portal tract; c, central vein, S, hepatic sinusoid. Bars denote 126, 79, 63, 15, 7 or 5 μm magnification, as indicated.
Figure 2.

Immunohistochemical analysis of adhesion molecules in the biopsy specimen. (A) Staining for ICAM-1 revealed that ICAM-1 expression was high on LSECs and that clusters of ICAM-1-positive hepatocytes were located around the central vein (arrowheads). (B) Many LFA-1-positive lymphocytes were present throughout the liver parenchyma and around the central zonal necrosis. Serial sections revealed that membranous ICAM-1 expression correlated with the presence of LFA-1-positive lymphocytes (arrowheads) around the central zonal necrosis. (C) VCAM-1 expression was high on LSECs and in the central vein (arrowheads). Arrowheads denote reaction products. Original magnification: ×100 (left panel) and ×400 (right panel). Counterstaining was performed with haematoxylin.
Figure 3.

Electron microscopic findings of the needle liver biopsy specimen obtained 4 days after admission. (A–B) Liver sinusoids. (A) Low-magnification view of liver sinusoids (denoted as S). The sinusoidal spaces contain lymphocytes, which adhere in clusters to DCs. Partially disrupted liver sinusoidal endothelial cells (LSECs) and coagulation necrosis of hepatocytes (white arrow) are also visible. (B) High-magnification view of the attachment of a DC to a LSEC and a lymphocyte via pseudopods (white arrowheads). S, hepatic sinusoid; DC = dendritic cell, Ly = lymphocyte, LSEC = liver sinusoidal endothelial cell. (C–E) Central vein. (C) Low-magnification view of the central vein. The central vein exhibited characteristic central zonal necrosis with confluent hepatocyte dropout and red blood cells (indicated by black stars) with an extensive inflammatory infiltrate. These findings all indicate central vein endotheliitis. The black arrow indicates the disruption of the endothelial lining cells in the central space. DC = dendritic cell, e = endothelial cell, Ly = lymphocyte, M = macrophage. (D) High-magnification view of the central vein. The capillary endothelial cells display a characteristic disrupted cytoplasmic architecture with the formation of cytoplasmic “blebs”. The white arrowhead indicates detachment of the endothelial cells. The black bracket bar indicates the attachment of a lymphocyte to an endothelial cell via a pseudopod. Ly, lymphocyte; e, endothelial cell. (E) High-magnification view showing the attachment of a DC to an endothelial cell via a pseudopod (black bracket bar). Bars denote 5, 2 or 1 μm magnification.
Immunohistochemistry of the liver biopsy (Fig. 1d–h) showed that the immune cells infiltrating the hepatic sinusoids and central vein area were CD83+ DCs (Fig. 1d) and CD8+ cytotoxic T cells (Fig. 1e). CD4+ helper T cells (Fig. 1f), CD56+ natural killer cells (Fig. 1g) and CD20+ B cells (Fig. 1h) were also present but in much smaller numbers in the hepatic sinusoids around the central vein.
The patient was started on 40 mg/day prednisolone to control the hepatic inflammation that was contributing to the hepatocellular injury. Her aspartate and alanine aminotransferase levels started declining after prednisolone was initiated. Three weeks later, these levels had normalised. The patient received 2.5 mg/day prednisolone for 1 year.
3. Discussion
Our histochemical examination of a liver biopsy showed the presence of atypical histological features in a case of acute AIH, including prominent centrilobular necrosis and central vein and hepatic sinusoidal endotheliitis. Moreover, electron microscopy showed that DCs and lymphocytes were attached to disrupted LSECs in hepatic sinusoids and that DCs attached to LSECs via pseudopods in the central vein.
In hepatic allografts, endotheliitis of the portal and central veins associates with pseudopod formation and membrane fusion.[9] Similarly, our electron microscopy analyses showed that there was cytoplasmic fusion between the endothelial cells and the lymphocytes and DCs.
In this case report, we found that DCs interacted with lymphocytes and LSECs in the hepatic sinusoids. It is possible that the interaction between the lymphocytes and DCs was mediated by ICAM-1 on the mature DCs, which promotes LFA-1 affinity maturation by immunohistochemistry. This in turn enhances DC-T cell conjugation, homotypic T cell interactions and T cell proliferation.[10] Our immunohistochemical analysis also showed that the hepatic sinusoids around the central vein contained many DCs that were positive for CD83, a marker of DC activation. Many CD8+ T cells were also observed in this tissue. Since DCs have a marked capacity to modulate the immune functions of CD8+ T cells, our preliminary findings suggest that acute AIH associates with central vein and sinusoidal endotheliitis and that this endotheliitis is due to the subendothelial infiltration of DCs and their activation of cytotoxic T cells.[11]
The integrins LFA-1 and very late antigen-1 (CD49a/CD29) on DCs may play an important role in their firm adhesion to endothelial cells and their transmigration to sites of immune action.[8] Our immunohistochemical observations suggest that in acute AIH, the central vein and sinusoidal endothelial cells promote the activation of DCs.
Moreover, VCAM-1 expression was evident in hepatic sinusoids and the central vein. The spindle-shaped LSECs observed by electron microscopy in the current case further support the idea that VCAM-1 plays a role in inducing changes to the shape and structure of endothelial cells during transendothelial migration of leukocytes.[12]
It can be difficult to diagnose AIH when it presents abruptly as acute liver failure because some of the characteristic features of AIH, including high serum IgG and autoantibody levels, may be absent. Moreover, instead of observing the characteristic histological features of AIH (ie, interface hepatitis), atypical histological findings may be observed, including centrilobular necrosis and/or central vein endotheliitis.[13]
Our electron microscopic and immunohistochemical analyses suggest that findings of sinusoidal endotheliitis caused by sinusoidal DC and lymphocyte infiltration together with hepatocyte necrosis and degeneration are congruent with a diagnosis of AIH. The underlying molecular mechanism should be elucidated in the future.
Acknowledgments
The authors thank Mariko Ogi, Tomoko Yoshii and Natsuki Hata for technical assistance with immunohistochemistry and electron microscopy.
Author contributions
Conceptualisation: Hiroaki Yokomori.
Data curation: Makoto Obu, Takemichi Okada, Hitoshi Yamazaki.
Project administration: Hiroaki Yokomori, Makoto Obu.
Software: Hiroaki Yokomori.
Supervision: Masaya Oda.
Visualization: Masaya Oda.
Writing – original draft: Hiroaki Yokomori, Takayuki Uematsu.
Writing – original draft: Hiroaki Yokomori.
Writing – review and editing: Hiroaki Yokomori, Takayuki Uematsu, Makoto Obu, Masaya Oda, Takemichi Okada, Hitoshi Yamazaki.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, DC = dendritic cell, ICAM-1 = intercellular adhesion molecule-1, Ig = immunoglobulin, LFA = lymphocyte function adhesion molecule, LSEC = liver sinusoidal endothelial cell, VCAM-1 = vascular cell adhesion molecule-1.
This case report did not involve any active intervention and therefore ethics approval was waived. The patient consented to participate in this report.
The patient provided written informed consent for publication of this case report and the accompanying images.
There are no conflicts of interest to declare.
References
- [1].Manns MP, Czaja AJ, Gorham JD, et al. American association for the study of liver diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–213. [DOI] [PubMed] [Google Scholar]
- [2].Hubscher SG. Transplantation pathology. Semin Liver Dis 2009;29:74–90. [DOI] [PubMed] [Google Scholar]
- [3].Abe K, Kanno Y, Okai K, et al. Centrilobular necrosis in acute presentation of Japanese patients with type 1 autoimmune hepatitis. World J Hepatol 2012;4:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pongpaibul A, Venick RS, McDiarmid SV, et al. Histopathology of de novo autoimmune hepatitis. Liver Transpl 2012;18:811–8. [DOI] [PubMed] [Google Scholar]
- [5].Shi Y, Dong K, Zhang YG, et al. Sinusoidal endotheliitis as a histological parameter for diagnosing acute liver allograft rejection. World J Gastroenterol 2017;23:792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol 2017;17:30–48. [DOI] [PubMed] [Google Scholar]
- [7].Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010;207:1247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mathan TS, Figdor CG, Buschow SI. Human plasmacytoid dendritic cells: from molecules to intercellular communication network. Front Immunol 2013;4:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ludwig J, Batts KP, Ploch M, et al. Endotheliitis in hepatic allografts. Mayo Clin Proc 1989;64:545–54. [DOI] [PubMed] [Google Scholar]
- [10].Steinhoff G, Behrend M, Schrader B, et al. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3. Am J Pathol 1993;142:481–8. [PMC free article] [PubMed] [Google Scholar]
- [11].Thomson AW, Satoh S, Nüssler AK, et al. Circulating intercellular adhesion molecule-1 (ICAM-1) in autoimmune liver disease and evidence for the production of ICAM-1 by cytokine-stimulated human hepatocytes. Clin Exp Immunol 1994;95:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 2011;15:1607–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stravitz RT, Lefkowitch JH, Fontana RJ, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology 2011;53:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


