Abstract
The purpose of this study was to evaluate the impact of the body composition changes on patients’ long-term outcomes after endoscopic resection or surgery for mucosal gastric cancer.
This case-control study included 96 patients who underwent endoscopic resection or surgery after propensity score matching. Areas of fat and muscle measured on CT were compared between the 2 groups. The effects of the variables on disease-free and overall survival were assessed using Cox-regression analysis and Kaplan-Meier survival analysis.
The median overall survival of the surgical and endoscopic resection groups was 91.1 and 93.9 months (P = .080). Fat area was decreased significantly more after surgery (P < .001). The number of patients with sarcopenia was increased in the surgery group. Kaplan–Meier plot showed that overall survival was significantly correlated with post-treatment sarcopenia (P = .049).
CT-based body composition analysis was helpful to evaluate the change in fat and muscle areas after treatment of early gastric cancer. The losses of fat and muscle after treatment were negatively associated with the patient overall survival.
Keywords: body fat distribution, computed tomography, endoscopic mucosal resection, gastrectomy, stomach neoplasms
1. Introduction
Early gastric cancer (EGC) is gastric cancer that is confined to the mucosa or submucosa, irrespective of the presence of regional lymph node metastasis.[1] EGC offers excellent outcome with a greater than 85% 5-year survival.[2] Surgical resection is a conventional treatment for EGC and endoscopic resection was considered an alternative treatment method for surgical resection only for elderly patients and cancer of the upper stomach.[3,4] However, endoscopic resection is widely used for EGC as the proportion of EGC among stomach cancers has increased with the widespread use of screening endoscopy.[1,3] As the stomach is preserved after endoscopic resection, the chance of metachronous stomach cancer is higher than that after surgical resection. However, repeated endoscopic resection is possible.
Resection of the stomach can lead to changes in the body habitus of the patients due to changes in the anatomic structure of the gastrointestinal tract. Many studies have reported changes in the nutritional status, weight and composition of body fat and muscle before and after gastrectomy for stomach cancer.[5–11] Body weight and the body mass index (BMI) are easily accessible indexes for the evaluation of these body habitus changes, but they are insufficient to access the proportional volume of fat and lean tissues.[12] Recently, CT-based evaluation of body composition measuring fat and muscle area has been highlighted in malignant and non-malignant conditions.[13,14] It was reported that sarcopenia is associated with several harmful outcomes such as a high mortality rate, functional decline, a high rate of falls, and a high incidence of hospitalizations.[15] The volume and relative ratio of visceral fat and subcutaneous fat have also been reported regarding their importance and impact on the patient's quality of life, treatment outcome and prognosis.[16–18] However, changes in the body composition after endoscopic resection for EGC and comparison of the influence of gastrectomy and endoscopic resection to body habitus have not been evaluated. It is easy to predict that the degree of change in body composition will be different depending on whether gastrectomy is performed or not. Therefore, a retrospective study using previous CT examinations for CT-based body composition evaluation is useful to investigate changes in muscle and fat component before and after the treatment of stomach cancer and to know its role in the patients’ quality of life and prognosis.
The purpose of this study was to compare the changes in the body composition before and after treatment between surgery and endoscopic resection for mucosal gastric cancer through quantitative analysis using CT and to evaluate the impact of body composition changes on the patients’ long-term outcomes.
2. Materials and methods
This study was approved by the institutional review board (IRB number: KC17RISI0026), and the informed consent requirement was waived.
2.1. Patients
This study was a retrospective, case-control study. Between January 2007 and December 2009, a total number of 670 consecutive patients were treated for mucosal gastric cancer in my institution. Among them, 535 patients underwent surgery and 135 patients underwent endoscopic resection. Patients were included if they
-
(a)
were pathologically diagnosed with mucosal gastric cancer;
-
(b)
had contrast-enhanced abdomen and pelvis CT performed before treatment;
-
(c)
underwent follow-up contrast-enhanced CT within 3 months to 1 year after pre-treatment CT;
-
(d)
had no history of known another malignancy.
Of consecutive patients, 368 patients were excluded for the following reasons:
-
(a)
EGC with submucosal invasion (n = 236);
-
(b)
no pre-treatment CT (n = 30);
-
(c)
no post-treatment CT (n = 49);
-
(d)
presence of known another malignancy (n = 53).
Finally, 302 patients were included, and 253 and 49 patients underwent surgery and endoscopic resection, respectively. A flowchart showing the numbers of patients that were included or excluded is presented in Figure 1. To reduce the effect of selection bias and imbalance of baseline characteristics of the surgical and endoscopic resection groups, we performed propensity score matching with 4 factors, patient age, gender, location and size of the stomach cancer. One-to-one matching between 2 treatment groups was achieved using the nearest neighbour matching method and 98 patients consisting of 49 pairs patients after surgery and endoscopic resection were finally included in this study. The patients’ demographic characteristics, including gender, age, BMI, pathologic results and laboratory findings (hemoglobin, albumin and total protein), were collected from electronic medical records. Tumor recurrence was identified by review of the medical records. The patients’ survival data were obtained from the medical records of our institution and national cancer registry database.
Figure 1.
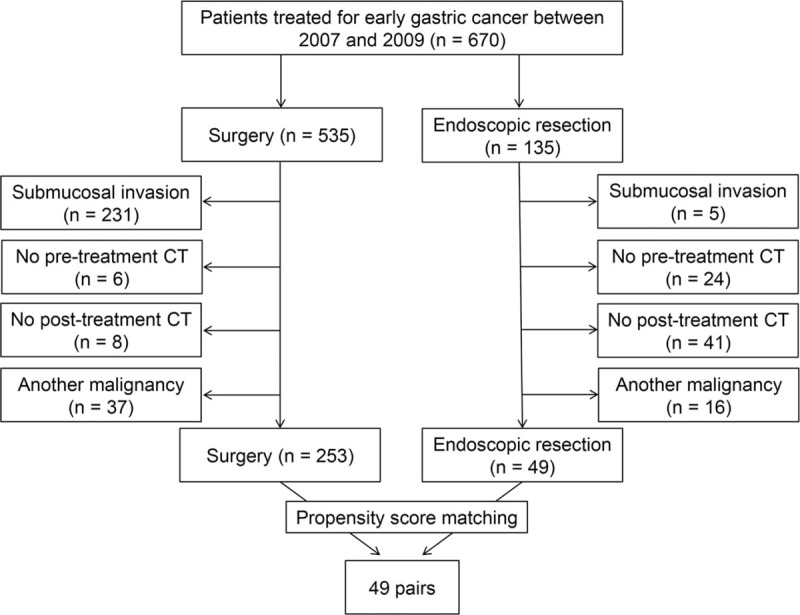
Flow chart of patient enrolment.
2.2. Follow-up endoscopy and CT
Endoscopy was performed to monitor the recurrence of the stomach cancer for patients who underwent either endoscopic resection or surgical resection. Board-certificated internal medicine faculty or clinical fellows performed endoscopy. Interval of endoscopy was variable from 6 months to 12 months according to the clinical decision. CT examinations were performed according to the physicians’ decisions. Patients after surgery and endoscopic resection underwent CT examinations with 6-month-interval and 1-year-interval, respectively.
2.3. CT protocol
CT examinations were performed using various machines (Somatom Definition/Somatom Definition AS+; Siemens Healthcare, Erlangen, Germany; Discovery CT750 HD, GE Healthcare, Milwaukee, WI, USA). Pre-treatment CT included early arterial and portal venous phases with the following parameters: tube voltage, 100 kVp and 120 kVp; 228 mAs and 248 mAs reference tube current. Delay time was determined using a bolus tracking method. Early arterial and portal phases were acquired with 8- and 36-s delays after achieving 100-Hounsfield units (HU) of attenuation of the descending aorta, respectively. Post-treatment CT consisted of only portal phase images (120 kVp, 180 mAs) obtained with a fixed 75-s delay after contrast injection. Iodinated contrast material (iopromide; Ultravist, Bayer AG, Berlin, Germany) was injected at a rate of 3.5 mL/s with a mechanical power injector. Axial images were reconstructed with a 5-mm thickness and a 5-mm interval.
2.4. Assessment of body composition
Portal phase images of pre- and post-treatment CT scans were used for body composition analysis with segmentation. A radiologist with 7 years of experience selected one axial image at the level of the transverse processes of the third lumbar spine. Each area of total body fat, visceral fat and subcutaneous fat, as well as abdominal circumference, was automatically measured on the selected axial image by a commercial workstation (TeraRecon Aquarius Workstation, TeraRecon, Foster City, California, USA). The workstation automatically distinguished subcutaneous and visceral fat using intersected muscle. The fat density ranged from -150 to -50 HU, and the muscle density ranged from 0 to 200 HU. The area of the skeletal muscle, including the abdominal wall and back muscles was measured semi-automatically using another workstation (Advantage Windows [AW] workstation 4.6, GE Healthcare, Milwaukee, Wisconsin, USA). The area of the muscle was defined as pixels with attenuation between -29 and 150 HU.[19] The radiologist who analyzed the images confirmed whether the automatically or semi-automatically selected area of each body component was appropriate.
Originally, diagnosis of sarcopenia required low muscle mass plus either low muscle strength or low physical performance and the diagnosis criteria was defined for aging patients.[20] However, many studies have performed CT-based body composition measurement and muscle area from CT has been used to define sarcopenia. In this study, we performed CT-based analysis and we defined sarcopenia using muscle area measured from CT.[14] To identify sarcopenia, the L3 skeletal muscle index (SMI) was calculated as the area of total skeletal muscle (cm2) at the level of L3 divided by the height squared (m2). The cut-off value for sarcopenia was referenced from a previous study performed for patients with solid tumors of the gastrointestinal tract; 52.4 cm2/m2 for men and 38.5 cm2/m2 for women.[13] Visceral obesity was defined as an area of visceral fat 100 cm2 or greater.[21] Classical measurement of obesity assessed by the BMI was also evaluated using 23 kg/m2 as the cut-off value for the Asian population.[22]
2.5. Statistical analysis
Differences in the baseline characteristics between the 2 treatment groups were evaluated using Student's t test and Chi-squared test for continuous and categorical variables, respectively. Laboratory findings and variables related to body composition measured before and after treatment were compared between the 2 groups using Student's t test. Changes in the variables after treatment versus baseline (%) were considered independent variables and were compared between treatment groups using Student's t test.
The time interval for disease-free survival was calculated as the time from treatment to tumor recurrence. Overall survival was defined as the time from treatment to death from any cause in expired patients and to the date of the last follow-up in living patients. Disease-free survival and overall survival according to treatment methods, visceral obesity and sarcopenia were compared using Kaplan–Meier analysis and the log-rank test. The effects of variables on disease-free survival and overall survival were assessed by Cox-regression analysis; continuous variables were included in analysis without cut off value. The hazard ratios (HRs) and 95% confidence intervals (CI) were calculated.
Data analyses were performed using SPSS 24.0 (IBM Corporation, Armonk, NY, USA) and R software (version 2.6.2, R Foundation for Statistical Computing, Vienna, Austria). P values less than .05 were considered statistically significant.
3. Results
All 96 patients with 48 pairs (F: M = 30: 66) were enrolled with a propensity score matching. Patients and lesion characteristics according to the treatment modality are shown in Table 1. The gender ratio, age, BMI and interval between pre- and post-treatment CT for the 2 groups were not significantly different. More than half of the cancer lesions were located in the mid portion of the stomach in the surgery group and upper portion in the endoscopic resection group (P = .004). The histologic type and differentiation of tumors were significantly different between the groups (each P < .001). Local tumor recurrence at the stomach was significantly higher in the endoscopic resection group than in the surgery group (P = .015). Hemoglobin, total protein and albumin were not significantly different between the 2 groups before and after treatment (Supplemental Digital Content 1).
Table 1.
Comparison of the characteristics of the patients and stomach cancer between the surgical and endoscopic resection groups.
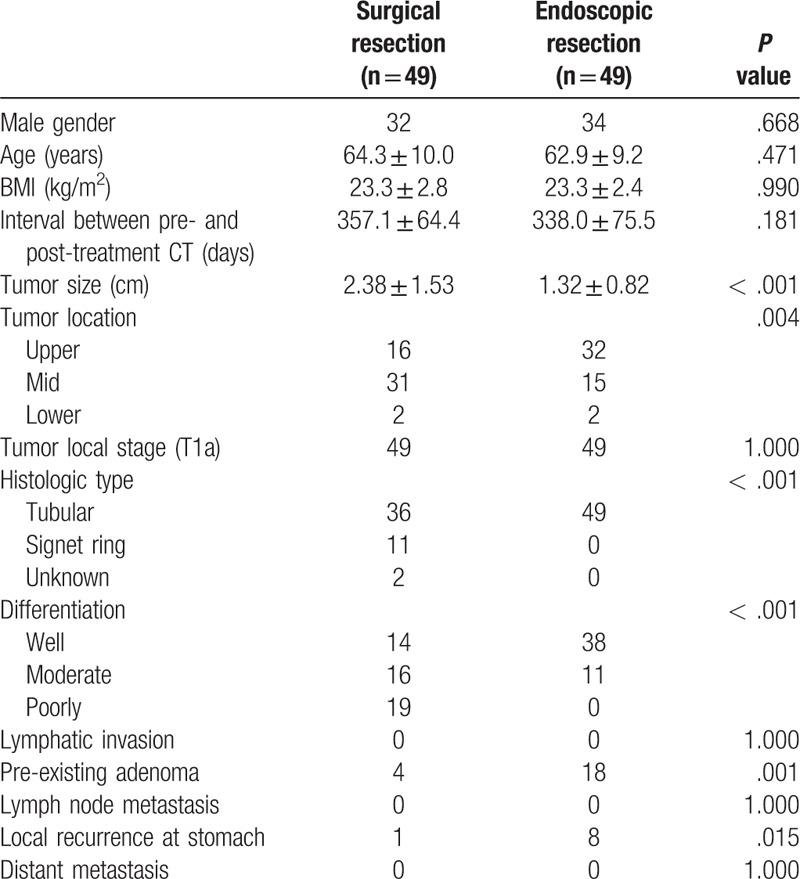
Changes in the body components are presented in Table 2. There was no significant difference in all variables between the groups before treatment. After treatment, areas of visceral fat, subcutaneous fat, and total fat, and abdominal circumference were statistically smaller in the surgical resection group than in the endoscopic resection group (P < .001). Only the area of muscle was not significantly different between the 2 groups after treatment (P = .182). Visceral fat, subcutaneous fat, total fat and abdominal circumference were decreased significantly in the surgical group and slightly increased in the endoscopic resection group (P < .001). The change in the area of muscle was not significantly different between the 2 groups (P = .429).
Table 2.
Comparison of the body composition changes before and after treatment between the surgical and endoscopic resection groups.
Twenty of 49 patients were sarcopenic before surgery, but the number of patients with sarcopenia increased up to 29 after surgery. In the endoscopic resection group, the number of patients with sarcopenia decreased from 19 to 16 of 49 patients after treatment (Supplemental Digital Content 2). The number of patients with obesity and visceral obesity decreased after surgery, but slightly decreased after endoscopic resection. The number of patients with sarcopenia and visceral obesity were not different between the 2 groups before treatment (P = 1.000 and .104) but those were significantly different after treatment between the 2 groups (P = .015 and < .001). Two representative cases show the difference in the change in the body composition after 2 treatments (Figs. 2 and 3).
Figure 2.
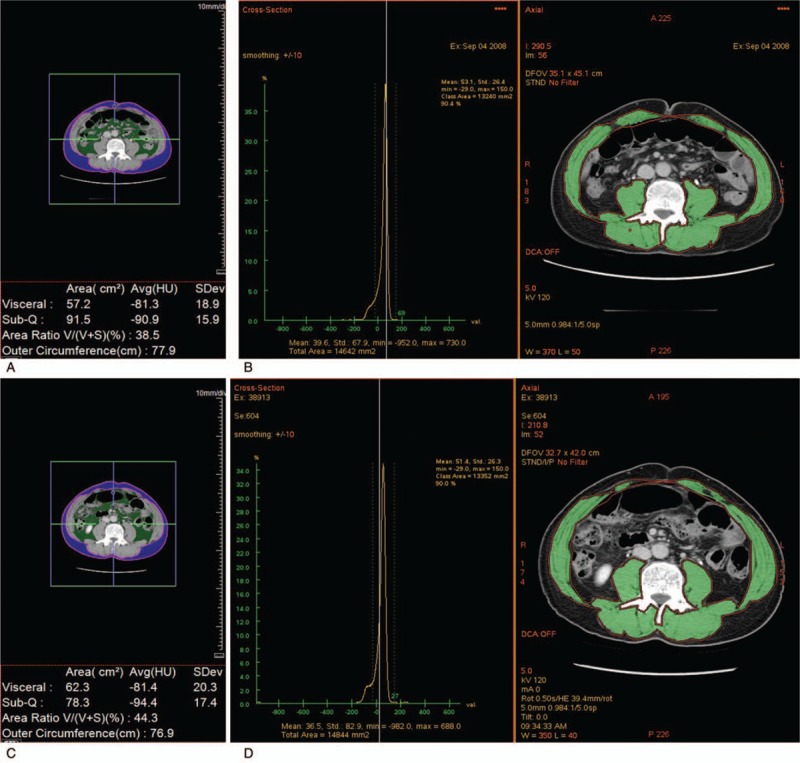
A 63-year-old man who underwent endoscopic submucosal dissection (ESD) of early gastric cancer. (a) The extent of visceral fat, subcutaneous fat and (b) muscle was measured on a transverse image at the level of the transverse processes of the third lumbar spine obtained before ESD (visceral fat, 57.2 cm2; subcutaneous fat, 91.5 cm2; muscle, 132.4. cm2). Compared with the extent acquired before ESD, (c) the extent of visceral fat and (d) muscle was slightly increased and that of subcutaneous fat was slightly decreased on a transverse image obtained at the same level after ESD (visceral fat, 62.3 cm2; subcutaneous fat, 78.3 cm2; muscle, 133.5 cm2).
Figure 3.
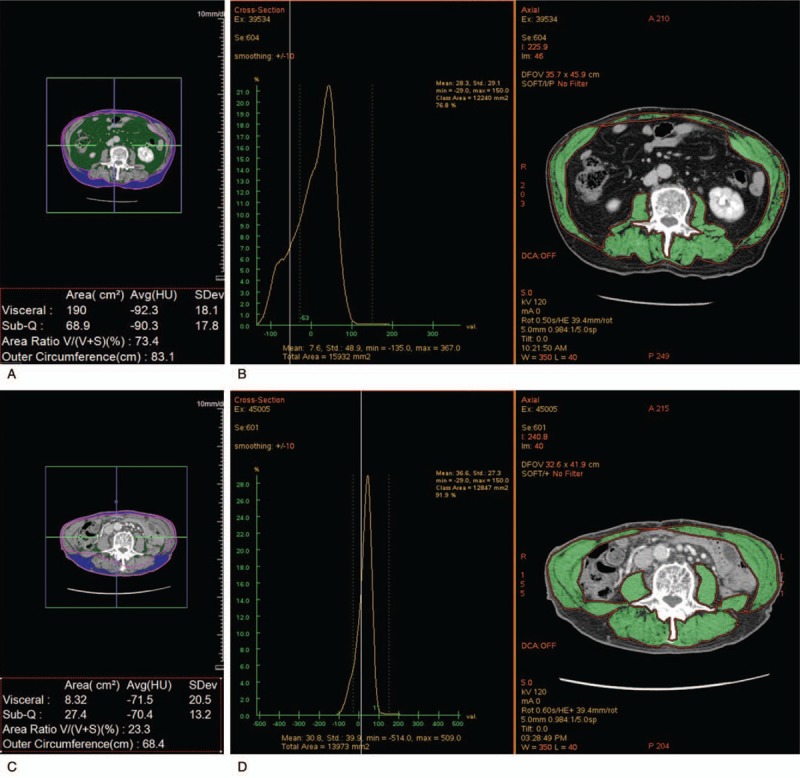
A 73-year-old man who underwent surgical resection of early gastric cancer. (a) The extent of visceral fat, subcutaneous fat and (b) muscle was measured on a transverse image at the level of the transverse processes of the third lumbar spine obtained before ESD (visceral fat, 190.0 cm2; subcutaneous fat, 68.9 cm2; muscle 122.4 cm2). Compared with the extents acquired before surgical resection, (c) the extent of visceral fat and subcutaneous fat was obviously reduced, and (d) the extent of muscle showed little interval change on a transverse image obtained at the same level after surgical resection (visceral fat, 8.3 cm2; subcutaneous fat, 27.4 cm2; muscle, 128.5 cm2).
During a median follow-up period of 90 months (range: 58–118 months), 6 patients died without EGC recurrence; 5 of them underwent surgery, and 1 underwent endoscopic resection. The causes of death were pneumonia, general weakness and dyspnoea in 3 patients, each respectively. The causes of death of the other 3 patients were unknown. The median disease-free survival of the surgical and endoscopic groups was 87.6 months (95% confidence interval, 105.2–113.8) and 91.1 months (114.7–119.3), respectively (P = .080). The median overall survival of the 2 groups was 91.1 months (110.8–115.9) and 93.9 months (97.5–114.8), respectively (P = .033).
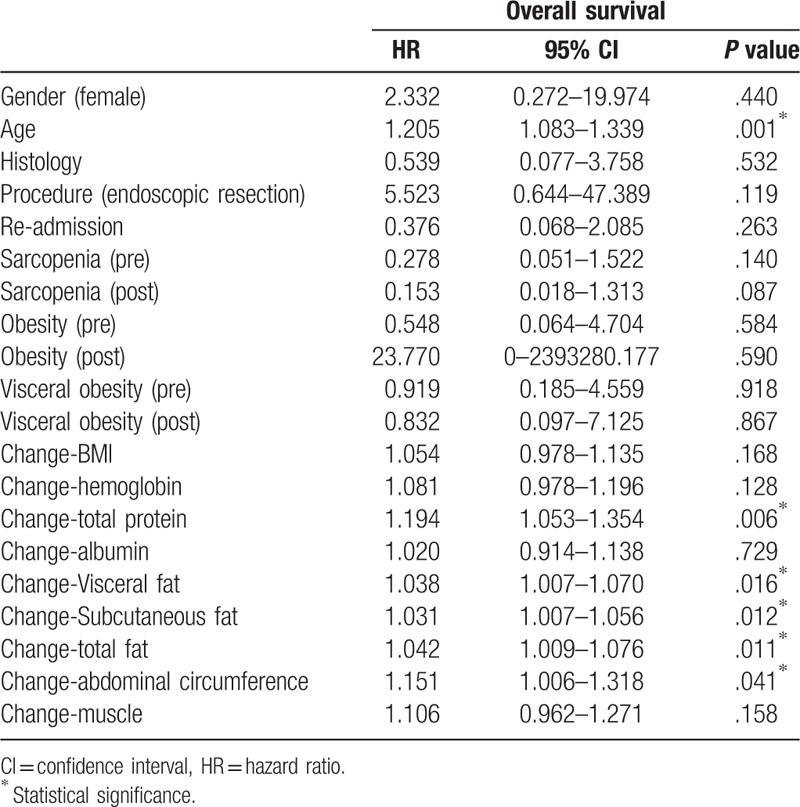
On Cox regression analysis to assess the association between the variables and tumor recurrence, the risk of tumor recurrence was increased with endoscopic resection and the presence of visceral obesity after the procedure (HR = 8.294 and 6.642) (Table 3). The risk of tumor recurrence tended to decrease with the increase in the subcutaneous and total fat, but the effects were negligible (HR = 0.984 and 0.985). The risk of death was slightly increased with older age and loss of in total protein, visceral fat, subcutaneous fat, total fat, and abdominal circumference (Table 4). Multivariate Cox regression was not performed because the numbers of tumor recurrence and death were small.
Table 3.
Univariate Cox regression analysis to assess the association between body composition factors and recurrence.
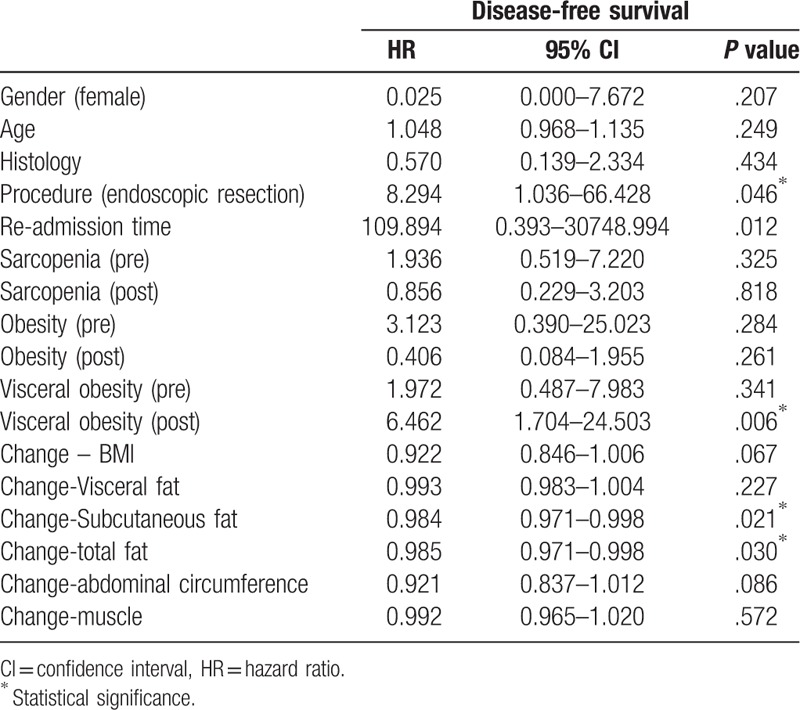
Table 4.
Univariate Cox regression analysis to assess the association between body composition factors and overall survival.
Kaplan-Meier analysis showed that disease-free survival was significantly shorter in patients with visceral obesity after treatment (P = .002). Disease-free survival was not associated with pre- and post-treatment sarcopenia and pre-treatment visceral obesity (P = .317, .818 and .332, respectively). Overall survival was significantly correlated with post-treatment sarcopenia (P = .049) and was not correlated with sarcopenia before treatment and visceral obesity before and after treatment (P = .115, .413, and .918, each respectively) (Fig. 4).
Figure 4.
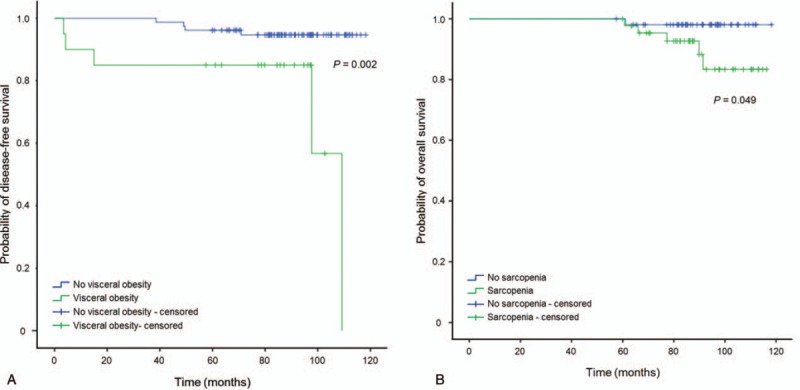
Kaplan-Meier analysis for disease-free survival and overall survival. (a) Kaplan-Meier analysis for disease-free survival showed visceral obesity after treatment correlated with poor recurrence-free survival of early gastric cancer patients (P = .002). (b) Kaplan-Meier analysis for overall survival showed that sarcopenia after treatment was correlated with the poor overall survival of early gastric cancer patients (P = .049).
4. Discussion
In this study, we compared body composition changes between surgery and endoscopic resection of EGC using quantitative analysis of CT. There were significant differences in body composition changes between the treatments. Many studies compared the oncologic outcomes between treatments, and the oncologic outcomes of endoscopic resection for EGC are comparable to those of surgery despite the drawbacks such as the lack of lymph node dissection and a high rate of metachronous cancer.[2–4] However, the change in the body composition after the 2 treatments and its impact on the long-term outcome are not well known. Several studies were interested in the change in the body composition and nutritional status after gastrectomy that removed total or part of the stomach.[5,11] However, there are few studies evaluating the changes in the body composition after endoscopic resection of EGC.
The strength of this study was that body composition analysis was performed based on CT. The different impacts of the 2 treatment methods for EGC on body composition were clarified objectively. Body composition can be assessed readily with accurate quantitative information from CT scanning acquired during diagnosis and follow-up.[23] As fat, muscle and bone have different ranges of density based on Hounsfield units (HU), they were distinguished from other tissues on CT. Therefore, CT is considered to be a gold standard for measuring body composition.[20] Many studies showed the utility of CT analysis of body composition in malignant tumors.[23,24] Detailed information about the fat and muscle composition using CT scan is useful to overcome the limitations of body weight or BMI.
This study showed similar results to those of a previous study that all fat tissue, including subcutaneous, visceral and total fat, was markedly decreased after surgery for stomach cancer.[11] Therefore, the proportions of obesity and visceral obesity were also decreased after treatment. By contrast, all variables concerning fat tissue were slightly increased after treatment in the endoscopic resection group, but the proportions of obesity and visceral obesity were slightly decreased. Paradoxical decrease in the proportions of obesity and visceral obesity despite the increase in the mean fat areas in the endoscopic group may be due to the combination of 2 phenomena, obese patients losing weight to the normal range and thin patients gaining weight to the normal range. We also evaluated the change in the muscle area after treatment of EGC. The change in the muscle area showed a similar tendency to change the fat areas in both groups; the muscle areas were decreased after treatment in the surgical resection group and were increased in the endoscopic resection group; however, the difference was not statistically significant.
Marked decrease in the fat and muscles areas after surgery may be due to a significant change in eating habits and indigestion resulting from gastrectomy.[5] An important consideration is that there is a high risk of developing sarcopenia in the surgery group than the endoscopic resection group. As the interval between pre- and post-treatment CT was not different between 2 groups, we could exclude the possibility of differences in body composition resulting from differences in recovery rates between 2 groups. Nutritional and metabolic changes and post-operative complications from the surgical procedure may affect the life style of the patients.[6] Surgery-related sarcopenia has been reported to be associated with postoperative morbidity and mortality in patients who underwent major abdominal surgery.[10,25] Sarcopenia is also associated with poor clinical outcome in patients undergoing cancer treatments.[13,23] The endoscopic resection group showed a slight increase in the muscle and fat areas. It may be due to no functional impairment by the preservation of the stomach as a digestive tract. An objective difference in the body composition between the treatment methods revealed in our study should be considered after treatment of EGC. Although this study could not show best time for post-treatment CT for body composition evaluation, a future study about sequential analysis of follow-CTs after EGC treatment may show the best time for post-treatment CT.
The additional strength of our study is that the long-term outcome of patients after EGC treatment was evaluated with the change in body composition. The risk of death was associated with age and a decrease in total protein, fat areas and abdominal circumference after treatment despite a low hazard ratio (< 1.3). Although a change in the muscle area was not associated with overall survival on Cox regression analysis, sarcopenia after treatment was a significant factor for overall survival on Kaplan-Meier analysis. The discrepancy in results of Cox regression and Kaplan-Meier analysis could be explained that change in muscle area was analyzed as a continuous variable in Cox regression analysis and post-treatment sarcopenia was analyzed as a nominal variable in Kaplan-Meier analysis. Therefore, the degree of fat loss resulting from nutritional impairment increased the risk of death proportionally, and significant muscle loss to sarcopenia after treatment was associated with death. Although we could not evaluate the impact of variables on patient death in each treatment group due to the small number of events, we should be concerned with pronounced changes in the body composition in patients after surgery compared with those after endoscopic resection. The results that all expired patients showed no recurrence of EGC also supported the importance of the change in body composition to death than the recurrence of EGC. Follow-up CT examinations after treatment of EGC seem to contain additional information for predicting patients’ long-term outcome.
Endoscopic resection and visceral obesity after treatment elevated the risk of stomach cancer recurrence. Our study showed a higher local tumor recurrence rate after endoscopic resection than after surgical resection. As a high incidence of metachronous stomach cancer after endoscopic resection is well known, a significant increase in recurrence after endoscopic resection was understandable.[26] However, visceral obesity could serve as a compounding factor related to recurrence in this study, because more frequent visceral obesity after treatment was noted in the endoscopic resection group.
There are several limitations to this study. First, this study was a retrospective study and the number of study patients was small. As mucosal gastric cancer patients have a very good prognosis after treatment, we selected 2007–2009 as the study time to ensure a long-term follow-up. At that time, endoscopic resection had a narrow indication and tended to be performed in patients with severe underlying disease. Therefore, the number of patients who underwent endoscopic resection was much smaller than surgery and many eligible patients who underwent endoscopic resection were excluded because of another malignancy. Although the number of patients was small, we underwent propensity score matching to select patients. Therefore, the difference between the 2 patient groups would have been considerably reduced. Second, tumor size, location, histologic type and differentiation were different between the groups, although the tumor size and location were factors of propensity score matching. The endoscopic resection group tended to have a smaller tumor size, better histologic type and higher frequency of well differentiation of the tumor and higher frequency of pre-existing adenoma. This limitation was also due to eligibility criteria for endoscopic resection and the difference in the tumor characteristics was inevitable.
In conclusion, CT-based body composition analysis was helpful to evaluate the change in fat and muscle areas after treatment of EGC. The losses of fat and muscle after treatment were negatively associated with the patient overall survival.
Author contributions
Conceptualization: Moon Hyung Choi, Kyung Ah Kim, Seong Su Hwang, Jae Young Byun.
Formal analysis: Moon Hyung Choi, Kyung Ah Kim.
Funding acquisition: Kyung Ah Kim.
Project administration: Moon Hyung Choi, Kyung Ah Kim.
Writing – original draft: Moon Hyung Choi, Kyung Ah Kim.
Writing – review & editing: Seong Su Hwang, Jae Young Byun.
Moon Hyung Choi orcid: 0000-0001-5962-4772.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CT = computed tomography, EGC = early gastric cancer, HR = hazard ratio.
This study was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (grant number: NRF-2016R1D1A1B04935077).
The authors have no conflicts of interest to disclose.
References
- [1].Choi MK, Kim GH, Park DY, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 2013;27:4250–8. [DOI] [PubMed] [Google Scholar]
- [2].Chiu PW, Teoh AY, To KF, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 2012;26:3584–91. [DOI] [PubMed] [Google Scholar]
- [3].Wang S, Zhang Z, Liu M, et al. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015;10:e0144774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park CH, Lee H, Kim DW, et al. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc 2014;80:599–609. [DOI] [PubMed] [Google Scholar]
- [5].Katsube T, Konnno S, Murayama M, et al. Changes of nutritional status after distal gastrectomy in patients with gastric cancer. Hepatogastroenterology 2008;55:1864–7. [PubMed] [Google Scholar]
- [6].Liedman B. Symptoms after total gastrectomy on food intake, body composition, bone metabolism, and quality of life in gastric cancer patients--is reconstruction with a reservoir worthwhile? Nutrition 1999;15:677–82. [DOI] [PubMed] [Google Scholar]
- [7].Liedman B, Andersson H, Bosaeus I, et al. Changes in body composition after gastrectomy: results of a controlled, prospective clinical trial. World J Surg 1997;21:416–20. discussion 20–1. [DOI] [PubMed] [Google Scholar]
- [8].Tanaka K, Miyashiro I, Yano M, et al. Visceral fat changes after distal gastrectomy according to type of reconstruction procedure for gastric cancer. World J Surg Oncol 2013;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tanaka K, Takiguchi S, Miyashiro I, et al. Impact of reconstruction method on visceral fat change after distal gastrectomy: results from a randomized controlled trial comparing Billroth I reconstruction and roux-en-Y reconstruction. Surgery 2014;155:424–31. [DOI] [PubMed] [Google Scholar]
- [10].Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol 2016;23:556–64. [DOI] [PubMed] [Google Scholar]
- [11].Yoon DY, Kim HK, Kim JA, et al. Changes in the abdominal fat distribution after gastrectomy: computed tomography assessment. ANZ J Surg 2007;77:121–5. [DOI] [PubMed] [Google Scholar]
- [12].Nattenmuller J, Wochner R, Muley T, et al. Prognostic Impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. PLoS One 2017;12:e0169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- [14].Malietzis G, Aziz O, Bagnall NM, et al. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol 2015;41:186–96. [DOI] [PubMed] [Google Scholar]
- [15].Beaudart C, Zaaria M, Pasleau F, et al. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010;59:341–7. [DOI] [PubMed] [Google Scholar]
- [17].Ladoire S, Bonnetain F, Gauthier M, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist 2011;16:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Y, Qiu Y, Thai T, et al. Applying a computer-aided scheme to detect a new radiographic image marker for prediction of chemotherapy outcome. BMC Med Imaging 2016;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–22. [DOI] [PubMed] [Google Scholar]
- [20].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].The Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity New criteria for ‘obesity disease’ in Japan. Circ J 2002;66:987–92. [DOI] [PubMed] [Google Scholar]
- [22].WHO expert consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [23].Gibson DJ, Burden ST, Strauss BJ, et al. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr 2015;69:1079–86. [DOI] [PubMed] [Google Scholar]
- [24].Mazonakis M, Damilakis J. Computed tomography: what and how does it measure? Eur J Radiol 2016;85:1499–504. [DOI] [PubMed] [Google Scholar]
- [25].Otsuji H, Yokoyama Y, Ebata T, et al. Surgery-related muscle loss and its association with postoperative complications after major hepatectomy with extrahepatic bile duct resection. World J Surg 2017;41:498–507. [DOI] [PubMed] [Google Scholar]
- [26].Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015;47:1113–8. [DOI] [PubMed] [Google Scholar]


