In the article, “Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial”,[1] which appeared in Volume 97, Issue 45 of Medicine, the disclosure statement should be “The authors report no other conflicts of interest.”
In Figure 5, the label for the first group should be HIV+ART+” instead of “HIV+ART−“.
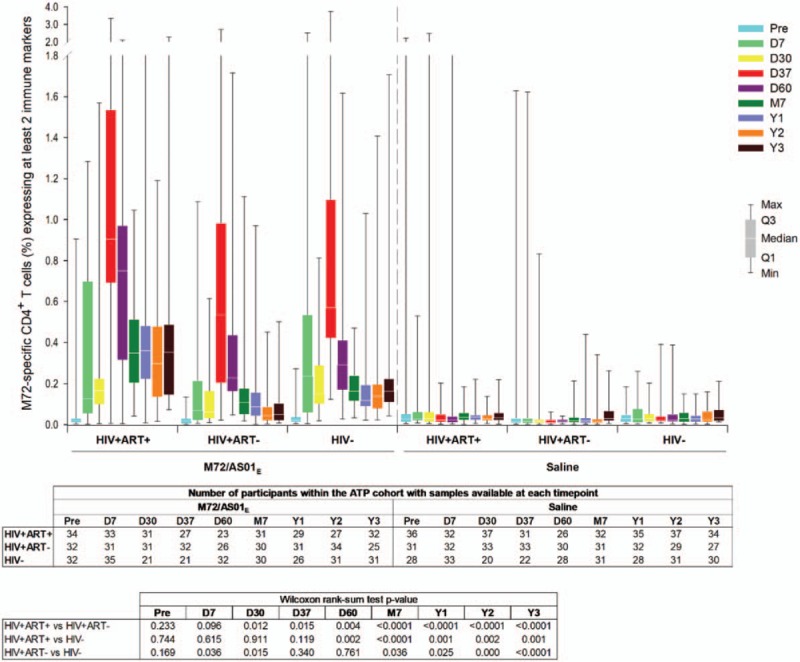
In the caption for Figure 6, “D6 = 30 days post-dose 2” should be “D60 = 30 days post-dose 2”.
Reference
- [1].Kumarasamy N, Poongulali S, Beulah FE. Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial. Medicine. 2018. 97:e13120. [DOI] [PMC free article] [PubMed] [Google Scholar]


