Abstract
Rationale:
The introduction of immune check-point inhibitors (ICIs) in the treatment of solid neoplasms is associated with the need to know and manage a new type of side effects that are commonly defined immune-mediated adverse events. Dermatologic immune-mediated adverse events are relatively common. Vitiligo-like lesions, defined as hypopigmented skin lesions, have already been associated with the use of ICIs in particular in patients with malignant melanoma, probably due to a common autoimmune mechanism against both melanoma cells and normal melanocytes. The onset of vitiligo-like lesions is very rare in non-melanoma patients and nowadays only few cases are described in the literature.
Patient concerns:
We described the case of a heavily pre-treated woman affected by renal cell carcinoma that has been treated with nivolumab for 2 years obtaining a stabilization of disease after an initial mild progression. After 9 months from the beginning of nivolumab, when the disease has reached its maximum stabilization, the patient developed vitiligo-like lesions of the back win halo nevi.
Diagnoses:
Vitiligo like lesion of the back not pre-existing before nivolumab treatment. The etiology was assumed to be nivolumab related as a result of an autoimmune activation against normal melanocytes.
Interventions:
The patient was followed with dermatological evaluations without changes in nivolumab dose and schedule
Outcomes:
No variations of the described lesions were recorded after the first description. The patients underwent a durable stabilization of her tumor.
Lessons:
This case on the one hand is the first case of vitiligo-like lesions associated with ICIs in patients affected by renal cell carcinoma, and on the other hand it seems to confirm that the onset of immumomediate adverse reactions, but in particular vitiligo lesions, can probably be considered a sign of response to immunological treatments probably as a consequence of activation of the immune response.
Keywords: immune checkpoint inhibitors, immunotherapy, renal cancer, vitiligo, vitiligo-like lesions
1. Introduction
Nivolumab is one of the new effective drugs that have recently implemented the treatment options for metastatic renal cell carcinoma (RCC). Nivolumab is a programmed cell death (PD-1) immune checkpoint inhibitor (ICI) antibody and it is now indicated as monotherapy for the treatment of advanced renal cell carcinoma after prior treatment with antiangiogenic therapies. This approval was the result of a Phase III trial that showed a survival benefit in patient treated with nivolumab compared to Everolimus after disease progression to other antiangiogenic agents.[1]
Nowadays nivolumab is widely used as it is approved for the treatment of other common metastatic neoplasms as melanoma and non-small-cell lung cancer.
It is actually well known that nivolumab, like all immune checkpoint inhibitors, is associated with dermatologic immune-mediated adverse events, such as skin rash, pruritus, vitiligo, and lichenoid skin reactions.
Vitiligo, that is defined as the appearance of hypopigmented skin areas, is an autoimmune skin disorder that originates from the loss of functional melanocytes of epidermis. Vitiligo or more in general vitiligo-like lesions, which include all the clinical manifestations similar to classical vitiligo, have been recognized to occur spontaneously or during anticancer treatments in patient affected by malignant melanoma. It is estimated that the risk of development of vitiligo is 10-fold higher in patients with melanoma, not only under treatment, than in the general population.[2]
One of the main explanations about the relationship between melanoma and vitiligo-like disorders is the immune activation against melanoma-associated antigens expressed by normal melanocytes as a result of a cross-reaction from melanoma cells that share the same antigens. Antigen release by anticancer therapies could explain a breakdown of immune tolerance to self-antigens expressed in normal cells or in benign lesions like nevi causing vitiligo like disorders.[2] This mechanism could be at the basis of the correlation between the onset of vitiligo-like disorders and outcome of those patients. In fact, the occurrence of depigmentated areas has been also associated with clinical benefit in patients treated with immunotherapy, not only ICIs, for advanced melanoma.[3]
Vitiligo-like disorders are absolutely rare in patients with, or treated for non-melanoma malignancies. To the best of our knowledge, no vitiligo-like disorders are reported in patients affected by renal cancer treated with ICIs.
We prospectively followed 25 patients treated with nivolumab inside the multicentric Italian Early Access Program.[4] These patients were followed with blood samples and clinical evaluations.
Herein, we describe the case of a woman treated with nivolumab that developed vitiligo-like disorders with halo nevi of the back and was referred to our dermatologic unit. This patient underwent a durable stabilization of her tumor, until then rapidly progressive.
2. Case presentation
A 66-year-old woman affected by metastatic renal cell carcinoma came to our Institution in December 2015 to start a therapy with nivolumab inside the national Early Access Program. She had a long history of treatments for her tumor before, started with a right nephrectomy in 2007 for a Grade 2 clear cell carcinoma (pT3N0).
She also underwent an adjuvant radiotherapy in right kidney area after surgery.
Radiological follow up was negative until June 2009 when a magnetic resonance (MR) scan showed 2 hepatic lesions that were increased at a subsequent computed tomography (CT) scan. In November 2009 the patient underwent to hepatic trans-arterial chemoembolization of these 2 lesions.
In February 2010 another CT scan showed 4 new hepatic lesions that were surgically removed with wedge resections in May 2010, but in February 2011 she experienced further liver progression and new small lung metastases appeared.
In February 2011 the patient started a first line sunitinib treatment obtaining a durable stability of disease until July 2013 when hepatic progression occurred.
From August 2013 to December 2013 a second-line treatment with sorafenib was administered but no control of hepatic lesions was obtained. In January 2014 a third line therapy with Everolimus was started and continued until October 2015 when RM and CT scans showed hepatic and lung progression.
In December 2015 the patient referred to our institution to be treated with Nivolumab inside the national Early Access Program. She started nivolumab at a standard dose of 3 mg/Kg biweekly that was performed for 45 infusions.
The liver lesions of the patients underwent an initial slight progression with an increase of few millimeters but from the ninth to the eighteenth month we registered a clear stabilization of the lesions. Lung metastases did not change significantly during the first 18 months of nivolumab treatment.
During the treatment period with nivolumab, the patient noticed, after about 9 months from the beginning of nivolumab, the appearance of clear spots of the back. She was referred to the dermatology service of our institution that diagnosed vitiligo-like areas of the back associated with halo nevi (Fig. 1). Diagnosis was performed on a clinical basis, no biopsy was deemed necessary by the dermatologist. Clinical evaluations and collection of medical history excluded pre-existing autoimmune disorders. No familiarity for vitiligo or autoimmune disorders were found. These features together with the age of onset, not typical for a classical vitiligo, and the causal relationship with nivolumab treatment have made the diagnosis of nivolumab related vitiligo like disorders the most likely and other causes of vitiligo were excluded.
Figure 1.
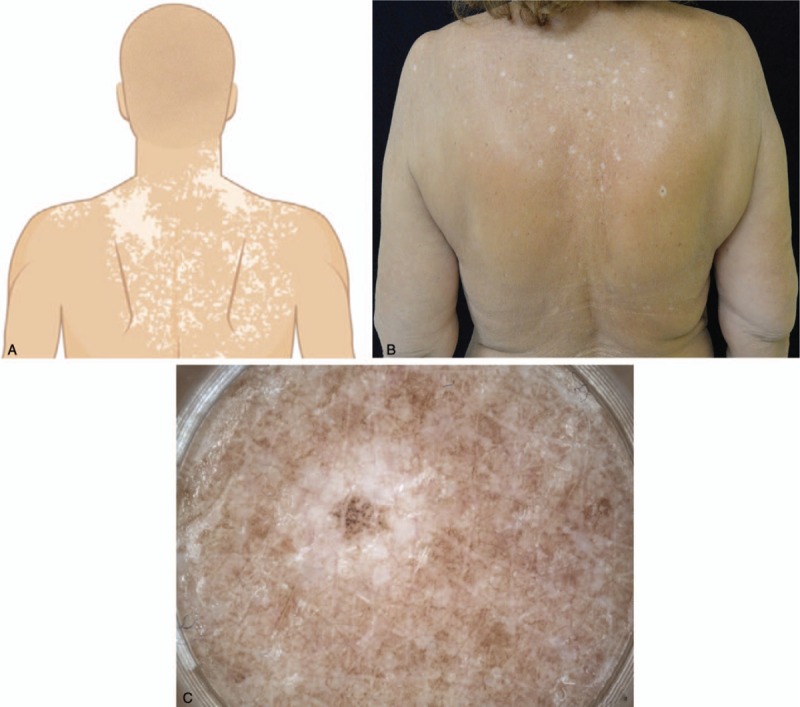
A: Pattern of depigmentation characterizing vitiligo-like lesions of high back occurring in patients receiving anti-programmed cell death-1 therapies. B: Vitiligo-like lesions of high back, sun-exposed area containing pre-existing actinic lentigos, after 9 months of treatment with nivolumab for advanced multi-treated renal neoplasia in a 66-year-old woman. C: Halo nevus of right scapula: pigmented flat lesion surrounded by a rim of depigmentation.
These areas persisted also at the following dermatologic evaluations without particular changes.
In December 2017, radiological evaluations showed a clear liver and lung progression and nivolumab was permanently discontinued. The patient's informed consent has been obtained.
3. Discussion
We reported the case of a patient with metastatic heavily pre-treated renal tumor who achieved a long-term stabilization of disease with nivolumab. During the treatment, vitiligo-like areas and halo nevi of the back were noticed and interestingly the onset of vitiligo-like lesions was contemporary with the stabilization of the metastatic disease.
Vitiligo is an autoimmune disorder that is estimated to affect 0.5% to 1% of the general population.[5] It is often associated with other autoimmune diseases, such as thyroiditis and rheumatoid arthritis.
To date there are numerous data about the correlation between malignant melanoma and the development of vitiligo. It is reported that about 20% of patients affected by melanoma present with vitiligo.[6]
A melanoma-specific autoimmune mechanism mediated by T-cells that infiltrate tumor and hypopigmented lesions may be at the base of vitiligo. Antibodies against melanoma-associated antigens such as MART-1 and gp100, which are present also in normal melanocytes, are probably likewise involved in the development of vitiligo in melanoma patients.[7] Cui et al have shown that in patients with melanoma and in patients with vitiligo, antibodies to human melanocyte antigens are present in a much higher percentage than in the control group (more than 80% for melanoma or vitiligo versus 7%).[8]
Another mechanism may be based on autoantibodies against Tyrosinase-Related Protein-2 (TRP-2) expressed by normal melanocytes.[9]
Immunotherapies for metastatic melanoma (ICIs, interleukin-2 but also other biological agents like tumor necrosis factor-α inhibitors) may induce vitiligo-like lesions probably enhancing the release of antigens potentially recognized by immune response.[10–12]
The spectrum of vitiligo-like lesions includes halo nevi that are usually observed around congenital or acquired melanocytic nevi. The clinical manifestation of halo phenomenon is characterized by progressive lightening of the color, disappearance of central nevus afterwards, and persistence of hypopigmentation. The etiology of halo nevi is unknown. As seen in vitiligo, melanocytes in the epidermis in the halo component of the nevus are completely absent, suggesting a similar etiologic mechanism.
Numerous studies have attempted to unravel the immunologic mechanisms by which an immune response develops to existing aggregates of nevus cells. The infiltrating cells are predominantly T-lymphocytes, and cytotoxic (CD8) lymphocytes outnumber helper (CD4) lymphocytes by a ratio of approximately 4:1. These, as well as scattered macrophages, comprise most inflammatory cells in halo nevi. The exact role that the lymphocytes play in the regression of halo nevi has not been fully determined, although a theory of direct cytotoxic effects on melanocytes seems plausible.[13]
Larsabal et al have recently evaluated clinical and biological features of vitiligo-like lesions associated with immune checkpoint inhibitors, comparing them with those of not ICIs related vitiligo. They reported that serum levels of CXCL10 (C-X-C motif ligand 10), a ligand of CXCR3 (C-X-C motif receptor 3) were significantly higher in patients with vitiligo-like lesions than in patients with vitiligo and in control subjects. Moreover, in patient treated with ICIs that developed vitiligo-like lesions, these are characterized by a higher proportion of skin CD8 T cells producing interferon-γ (IFN-γ), or both IFN-γ and tumor necrosis factor α (TNFα), compared with T cells isolated from perilesional skin of patients with classical vitiligo or healthy skin.
Clinical features in these 2 groups are also different. Vitiligo like lesions are characterized by multiple flecked white macules localized on photo-exposed areas while classical vitiligo is characterized by white patches generally symmetrical distributed and localized on face, hands, elbows, and trunk.[5]
Clinical features of vitiligo-like lesions developed by our patient described in this paper perfectly match those above described, endorsing the hypothesis of more typical pattern of vitiligo when it is associated with treatment with ICIs.
Association between the development of hypopigmented lesions and non-melanoma malignancies and between vitiligo-like disorders and immunotherapies for tumor types other than melanoma is extremely rare.
To date the development of vitiligo-like disorders during treatment with ICIs has been described in a patient treated with nivolumab for lung cancer, in a man treated with nivolumab for an acute myeloid leukemia, in a young woman treated with nivolumab for a metastatic soft tissue sarcoma and in a man treated with nivolumab plus ipilimumab for an advanced hepatocellular carcinoma.[14–17]
Vitiligo-like lesions occurrence during immunotherapy has been associated with better prognosis in term of higher objective tumor response rates and significantly improved progression-free and overall survival in patients with melanoma. Signs of tumor response were reported in almost all the cases reported above.[18]
The patient described in this paper was treated with nivolumab for RCC for about 2 years obtaining a durable stabilization of her tumor until then rapidly progressing. If we consider that the patient treated with nivolumab for acute myeloid leukemia, was treated in a maintenance setting, we can conclude that all patients treated with ICIs (in particular with nivolumab) for non-melanoma malignancies that developed vitiligo-like disorders experienced benefit from anticancer therapy as of those reported in the literature.
The current report had some limitations. For example, no biopsies were performed at the onset of vitiligo-like areas because the diagnosis was based on clinical features. Additionally, no data about the long-term persistence of the skin disorder was obtained because the patient continued anticancer therapies (cabozantinib) elsewhere.
There is an urgent need for prognostic and predictive biomarkers in the landscape of the treatment for renal cell carcinoma and the evaluation of immune system activation is one of the most studied fields. For example, many inflammatory indices, easy to obtain from the count of immune-inflammatory circulating cells (neutrophils, lymphocytes, and platelets), have been evaluated for this purpose, but actually, none of them are used in clinical practice.[19–20]
The possibility to correlate also the onset of immune-related adverse events with outcome is challenging.
Although a correlation between the onset of vitiligo-like disorders and outcome may be observed in all the few cases to date described in literature, actually we obviously cannot consider these signs as a reliable indication of tumor response in non-melanoma patients treated with ICIs. Despite this, new reports to correlate the occurrence of vitiligo-like disorders, and more in general immune-mediated cutaneous events with tumor responses may help to increase the predictive power of these signs that are moreover extremely simple to evaluate.
Author contributions
Investigation: Michela Ricci.
Methodology: Ugo De Giorgi and Ignazio Stanganelli.
Project administration: Alessia Filograna.
Supervision: Cristian Lolli, Matelda Medri, Ugo De Giorgi, and Ignazio Stanganelli.
Validation: Ugo De Giorgi.
Writing – original draft: Cristian Lolli and Matelda Medri.
Writing – review & editing: Cristian Lolli, Michela Ricci, Giuseppe Schepisi, Alessia Filograna, Ugo De Giorgi, and Ignazio Stanganelli.
Footnotes
Abbreviations: CD4 = cluster of differentiation 4, CD8 = cluster of differentiation 8, CT = computed tomography, CXCL10 = C-X-C motif ligand 10, CXCR3 = C-X-C motif receptor 3, gp100 = glycoprotein 100, ICI = immune checkpoint inhibitor, IFN-γ = interferon-γ, MART-1 = melanoma-associated antigen recognized by T cells 1, MR = magnetic resonance, PD-1 = programmed cell death-1, RCC = renal cell carcinoma, TNFα = tumor necrosis factor α, TRP-2 = Tyrosinase-Related Protein-2.
Ugo De Giorgi and Ignazio Stanganelli jointly supervised this work as co-senior authors. The authors have no conflicts of interest to disclose.
References
- [1].Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schallreuter KU, Levenig C, Berger J. Vitiligo and cutaneous melanoma. A case study. Dermatologica 1991;183:239–45. [DOI] [PubMed] [Google Scholar]
- [3].Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 2006;354:709–18. [DOI] [PubMed] [Google Scholar]
- [4].De Giorgi U, Cartenì G, Giannarelli D, et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int 2018;Jun 29. [DOI] [PubMed] [Google Scholar]
- [5].Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol 2017;76:863–70. [DOI] [PubMed] [Google Scholar]
- [6].Lerner AB, Nordlund JJ. Should vitiligo be induced in patients after resection of primary melanoma. Arch Dermatol 1977;113:421. [PubMed] [Google Scholar]
- [7].Mandelcorn-Monson RL, Shear NH, Sambhara S, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol 2003;121:550–6. [DOI] [PubMed] [Google Scholar]
- [8].Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol 1995;131:314–8. [PubMed] [Google Scholar]
- [9].Okamoto T, Irie RF, Fujii S, et al. Anti-tyrosinase-related protein-2 immune response in vitiligo patients and melanoma patients receiving active-specific immunotherapy. J Invest Dermatol 1998;111:1034–9. [DOI] [PubMed] [Google Scholar]
- [10].Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol 2006;126:2658–63. [DOI] [PubMed] [Google Scholar]
- [11].Sibaud V, Robert C. Pigmentary disorders induced by anticancer agents. Part II: targeted therapies. Ann Dermatol Venereol 2013;140:266–73. [DOI] [PubMed] [Google Scholar]
- [12].Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol 1997;37:620–4. [DOI] [PubMed] [Google Scholar]
- [14].Uenami T, Hosono Y, Ishijima M, et al. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: a case report. Lung Cancer 2017;109:42–4. [DOI] [PubMed] [Google Scholar]
- [15].Yin ES, Totonchy MB, Leventhal JS. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: a novel finding. JAAD Case Rep 2017;3:90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dumbrava EI, Ivan D, Subbiah V. Hypopigmented skin lesions after immunotherapy. JAMA Oncol 2018;Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodríguez-Lomba E, Molina-López I, Suárez-Fernández R, et al. Vitiligo-like lesions and immune checkpoint inhibition therapy: is it truly an adverse event exclusive to patients with melanoma? Clin Exp Dermatol 2018;43:598–9. [DOI] [PubMed] [Google Scholar]
- [18].Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773–81. [DOI] [PubMed] [Google Scholar]
- [19].Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget 2016;7:54564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 2015;5:e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]


