Central Illustration.
Proposed Multifactorial Impact of Doxorubicin-Induced Endothelial Damage on Cardiomyocyte Health and Function
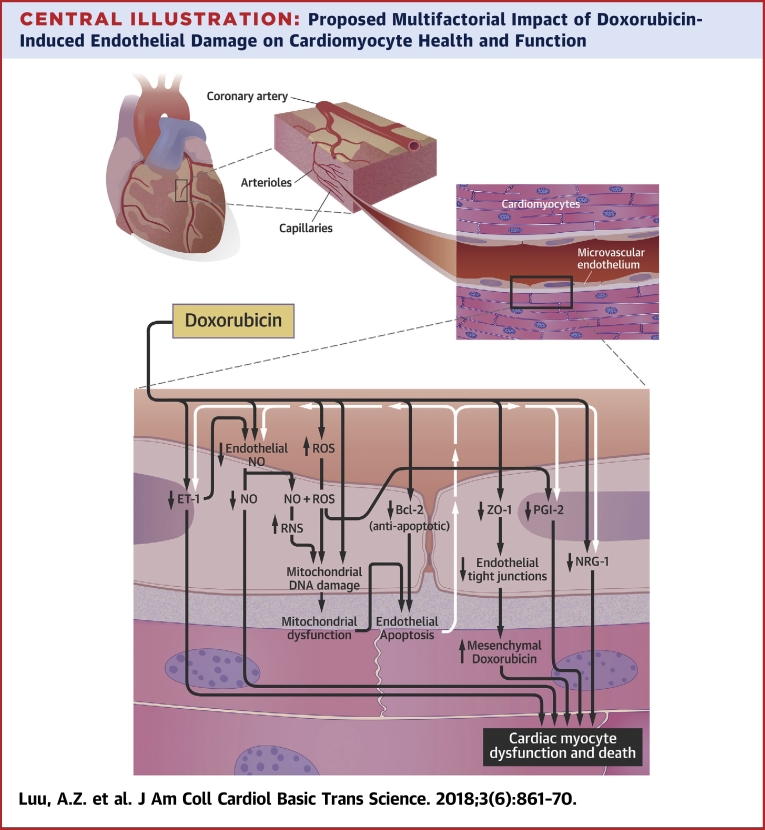
The endothelium-mediated cardiomyocyte supportive functions occur at the capillaries. Doxorubicin likely reduces tight junction formation by lowering the expression of zona occludens (ZO)-1, which can increase the levels of doxorubicin in cardiac tissues. Doxorubicin can decrease the levels of endothelium-derived neuregulin (NRG)-1 through an unknown mechanism and prostaglandin I2 (PGI2) via reactive oxygen species (ROS) generation. Doxorubicin is suggested to directly decrease the levels of nitric oxide (NO) in endothelial cells via enzymatic inhibition. There might also be an indirect decrease of NO through the reduction in endothelin (ET)-1 and elevation in ROS levels. Reactive nitrogen species (RNS) forms from the reaction between ROS and NO. RNS and ROS damage mitochondrial deoxyribonucleic acid (DNA). It is well established that doxorubicin induces mitochondrial DNA damage in an RNS/ROS-independent manner. The resulting mitochondrial dysfunction, along with a possible doxorubicin-mediated decrease in B-cell lymphoma (Bcl)-2, leads to apoptosis of the endothelial cells. The death of these cells can further reduce the availability of NO, ET-1, PGI2, and NRG-1 to the cardiomyocytes. The decline in NO, ET-1, PGI2, and NRG-1 release from endothelial cells and elevation in mesenchymal doxorubicin concentrations are postulated to increase the death and dysfunction of cardiac myocytes.