Figure 1.
Expression of Amyloid-β42 in the Drosophila Nervous System Generates Suboptimal Neurons that Upregulate flowerLoseB and azot
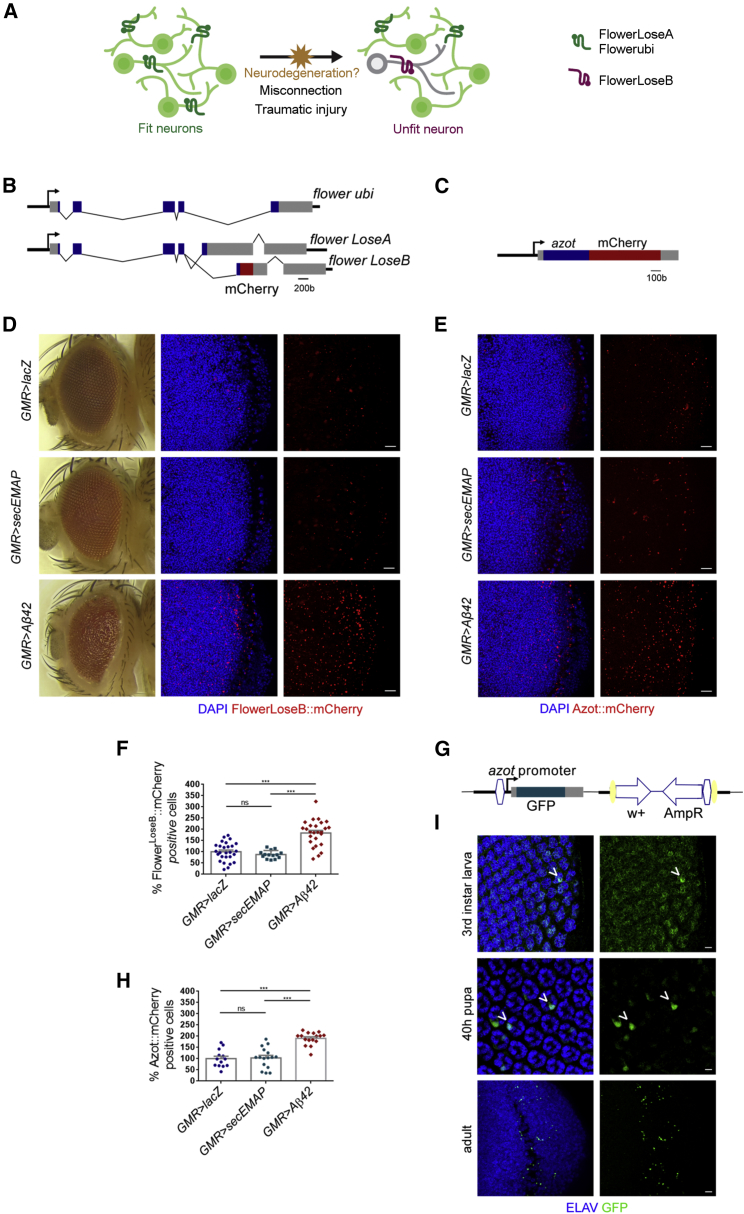
(A) Schematic illustrating neuronal fitness comparison. Neurons express ubiquitously Flowerubi or FlowerLoseA at their cellular membrane, but external insults such as a traumatic injury or failing to establish synaptic connections during development can decrease the fitness status of one neuron (or one subpopulation of neurons) that upregulate FlowerLoseB. We hypothesize that in the course of neurodegeneration induced by β-amyloid, neurons enter a period of suboptimality, characterized by a low fitness status and upregulation of the FlowerLoseB isoform.
(B) Representation of the flowerLoseB::mCherry reporter. Each flower isoform has a different last exon. Based on this particularity, we generated a reporter specific for flowerLoseB by introducing the mCherry sequence at the end of the exon specific for this isoform (exon 6). Blue rectangles are exons, the 5′ and 3′ UTRs are shown in gray, and the red box shows the localization of the mCherry tag (not to scale).
(C) Schematic of the azot::mCherry reporter that was obtained by fusion PCR. This construct includes 2,430 bp of the azot promoter region, the azot exon plus 175 bp of the 3′ end fused to mCherry (in red). The azot coding region is in blue, and UTRs are represented in gray.
(D) flowerLoseB::mCherry reporter (red) is strongly upregulated in the optic lobe of GMR > Aβ42 (amyloid-β42) adults, but not in the optic lobe of GMR>lacZ or GMR > secEMAP controls of the same age; the nuclear marker DAPI is shown in blue. Scale bar: 10 μm. The eye of GMR > Aβ42 flies shows a strong degenerative phenotype.
(E) azot::mCherry reporter (red) expressed in the optic lobe of adult flies in the presence of GMR-driven lacZ, secEMAP, or Aβ42; DAPI is shown in blue. Scale bar: 10 μm.
(F) Quantification of the percentage of FlowerLoseB::mCherry-positive cells in the optic lobes of the indicated genotypes. The number of FlowerLoseB::mCherry-positive cells detected for the GMR>lacZ control group was assumed to be 100%.
(G) Schematic of the modified azot{KO;GFP} locus. This transgenic line was generated by integration of a knockin construct containing the GFP sequence, under the control of the azot endogenous promoter, into the azot knockout locus. The 5′ and 3′ UTRs of the azot gene are shown in gray. The vector backbone was conserved in the knockin line (w+, AmpR). The yellow ellipses are loxP sites, and the white hexagons are attL regions.
(H) Quantification of the percentage of Azot::mCherry-positive cells in the optic lobe of the indicated genotypes. The number of Azot::mCherry-positive cells for the GMR>lacZ control group was assumed to be 100%.
(I) Eye imaginal discs of third-instar larva, retina of 40-hr pupa, and adult optic lobes of GMR > Aβ42 adults showing immunolabeling for the nuclear marker ELAV (blue) and endogenous GFP signal produced from azot{KO;GFP} (green). Arrowheads point to co-localization between ELAV and GFP. Scale bar: 5 μm.
Error bars show SEM. Ns, no significant. ∗∗∗p < 0.001. All genotypes are heterozygous. See also Figures S1 and S2.