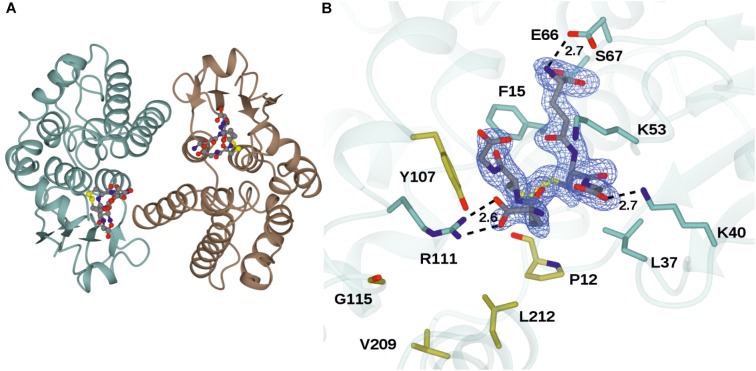
FIGURE 2.
Structures of GSTU25 and target residues. (A) Structure of the GSTU25 dimer, with monomers in blue and brown. Oxidized glutathione can be observed in each of the monomer active sites, in stick format. (B) Active site of the GSTU25 monomer showing binding of GSSG. The electron density corresponds to the Fo-Fc omit map contoured at a level of 3σ, and is that which was obtained prior to refinement of the ligand atoms, which have been added from the refined ligand complex for clarity. Side chains of residues conserved between U24 and U25 are shown with side-chain carbon atoms in Blue; Side-chains of residue positions chosen for mutation are shown with side-chain carbon atoms in gold.