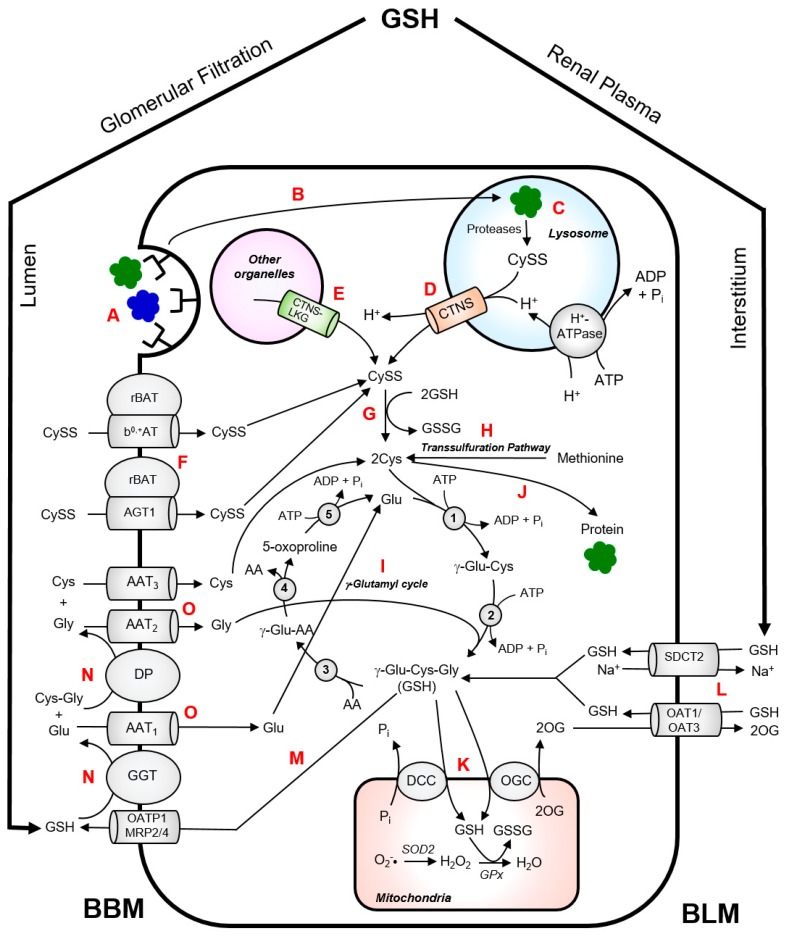
Figure 2.
Intermediary thiol metabolism in renal proximal tubular cells. CySS-containing proteins are reabsorbed from the brushborder membrane (BBM) of the PTCs via receptor-mediated endocytosis (A). The CySS-containing proteins enter the lysosomal compartment via fusion of protein-loaded transport vesicles with the lysosomes (B). These proteins undergo cathepsin-catalyzed degradation which generates CySS (C). Cystinosin facilitates the proton-driven efflux of CySS from the lysosomes into the cytosol (D). Cystinosin-LKG can also facilitate exodus of CySS from other cytosolic compartments (E). Non-protein CySS can be taken up by PTCs through heterodimeric CySS transporters, b0,+AT-rBAT and AGT1rBAT, and contributes to the cytosolic CySS pool (F). In the cytosol, CySS is rapidly reduced to Cys by cytosolic reducing systems (G). Alternatively, Cys can also be produced via trans-sulfuration pathway (H). The Cys generated in the cytosol can be used for the synthesis of GSH via γ-glutamyl cycle (I) or synthesis intracellular proteins (J). Most of the GSH in the mitochondrial matrix is obtained from the cytosolic GSH pool via oxoglutarate carrier (OGC) or dicarboxylate carrier (DCC) (K). In the mitochondria, superoxide radicals (O2−•) generated from oxidative phosphorylation are converted to hydrogen peroxide (H2O2) by superoxide dismutase 2 (SOD2). H2O2 is eventually neutralized to water (H2O) by glutathione peroxidase (GPx) which requires GSH as a cofactor. The kidney extracts approximately 80% of GSH from the plasma. Approximately 50% of this GSH enters the PTCs via sodium–dicarboxylate cotransporter 2 (SDCT2) and organic anion transporters 1 and 3 (OAT1/3) at the BLM (L). Glomerular filtration accounts for approximately 30% of the GSH extraction via BBM route. GSH can also undergo turnover by efflux into the lumen via either organic anion transporting polypeptide 1 (OATP1) or multidrug resistance proteins 2 and 4 (MRP2/4) (M). The high activity of γ-glutamyltransferase (GGT) and dipeptidase (DP) in the BBM of PTC facilitates degradation of GSH to its constituent amino acids (N). The constituent amino acids are then taken up by their respective transporters (AAT) (O) and are used for the cytosolic re-synthesis of GSH. Enzymes of the γ-glutamyl cycle: γ-glutamylcysteine synthetase (1); GSH synthase (2); γ-glutamyl transpeptidase (3); γ-glutamyl cyclotransferase (4); 5-oxoprolinase (5).