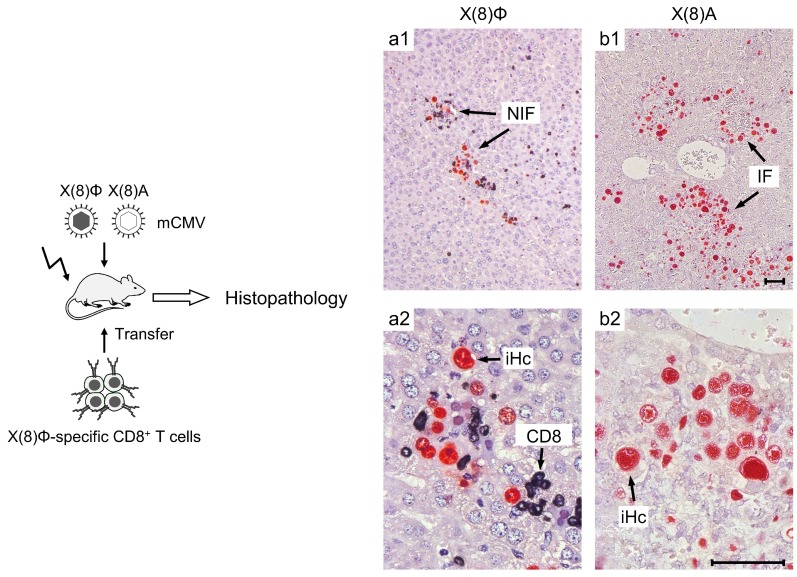
Figure 1.
Basic model of immunotherapy of CMV disease. (Left) Experimental protocol. Mice representing designated HCT recipients become immunocompromised by hemato-ablative total-body γ- irradiation (flash symbol), which is followed by infection and by transfer of CD8+ T cells, either CTLLs or cells ex vivo isolated from infected, immunocompetent donor mice, which are both specific for the same viral antigenic peptide (mostly a nonapeptide) with the general sequence X(8)Φ, where X represents amino acid residues and Φ represents the MHC-anchoring C-terminal residue that is mostly a hydrophobic residue. For demonstrating epitope specificity of protection, mice become infected either with parental mCMV encoding the functional antigenic peptide X(8)Φ or with the virus mutant that encodes the nonfunctional sequence X(8)A. The mutation strategy provides dual security, since X(8)A mostly does not even exist as a peptide, because it is usually not generated in the proteasome in the first place. In addition, in case X(8)A is generated, it would fail to anchor to an MHC molecule. (Right) Representative examples of liver histopathology after CD8+ T cell transfer and infection with either parental virus mCMV-X(8)Φ (a1, overview, a2, detail) or mutant virus mCMV-X(8)A (b1, overview, b2 detail). Bar markers represent 50 µm throughout. Immuno-histological staining identifies infected liver cells, which are primarily hepatocytes (iHC, red-stained) as well as tissue-infiltrating CD8+ T cells (CD8, black stained) NIF, nodular inflammatory focus. IF, focus of infection. The representative example is taken from Reference [79], PLOS Pathogens, 2015.