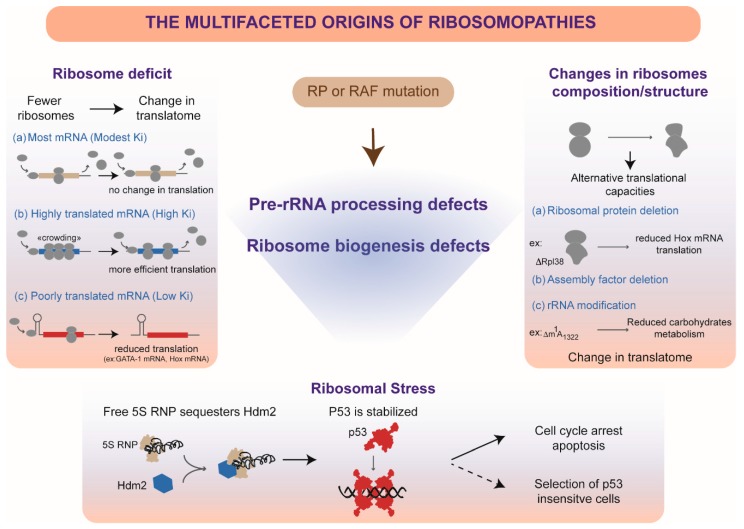
Figure 5.
The molecular effects of mutations affecting pre-rRNA processing and ribosome assembly. Mutations in genes encoding RPs or RAFs can lead to pre-rRNA processing or ribosome assembly defects. In human cells, different hypotheses may explain how these defects affect cell fate. Ribosome deficit corresponds to reduction in the number/concentration of ribosomes per cell. Depending on their translation initiation rate (Ki), mRNAs translation may be differently affected: (a) Translation of mRNAs that have a modest initiation rate will be mildly affected; (b) mRNAs having a high initiation rate may be more efficiently translated, as reduction in ribosome availability will reduce ribosome crowding and thus improve elongation efficiency. (c) In contrast, reduced concentrations of ribosomes will penalize translation of mRNAs that have low initiation rates [172]. Ribosome alteration refers to the ribosomes that are still produced despite ribosome assembly defects. Quality of these ribosomes (protein composition, RNA processing, and modification) may be suboptimal and change their translational properties, so that translation of specific mRNAs is not properly ensured or regulated. These different mechanisms are not mutually exclusive and may contribute in several ways to the variety of symptoms presented by patients suffering ribosomopathies. Ribosomal stress is induced by impairment of ribosome biogenesis, which promotes accumulation of free RPs that can assume alternative function. The 5S RNP particle, in which the 5S rRNA associates with RPs RPL5 and RPL11, plays a major role in this stress response. As a free complex, it binds and sequesters HDM2, the ubiquitin ligase that constitutively targets the tumor suppressor p53 for degradation. Inactivation of HDM2 promotes stabilization of p53, which regulates progression through the cell cycle. Chronic stimulation of p53 in ribosomopathies may also favor the selection of cells resisting p53 activity and prone to develop a tumorigenic program.