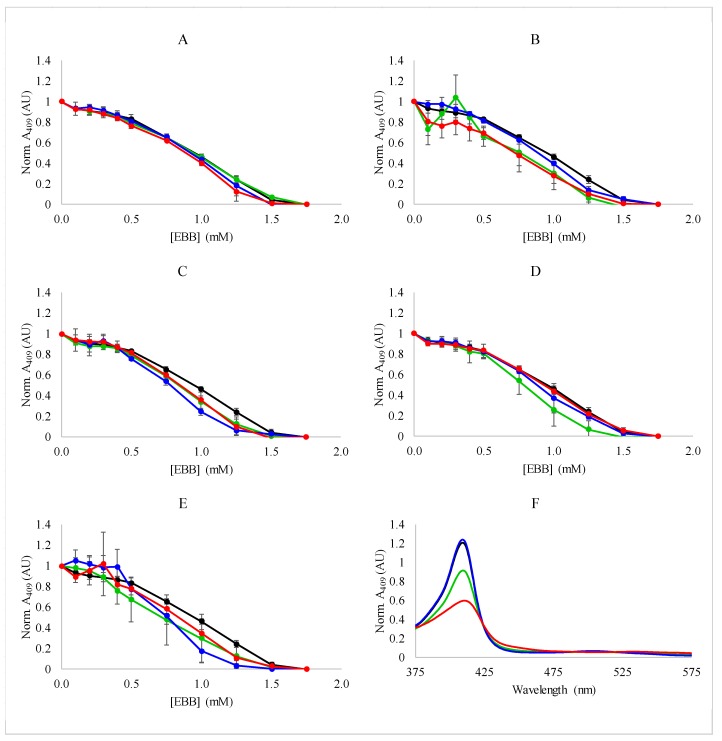
Figure 3.
Dissociation of heme from myoglobin monitored by absorbance spectroscopy—Denaturation of myoglobin (0.15 mg/mL) by EBB was monitored by the loss of heme absorbance at 409 nm. Experiments were performed in the presence of 0 (black), 9.4 mM (green), 28.1 mM (blue), 56.3 mM (red) ionic liquids or salts. (A) NaCl; (B) LiBF4; (C) BMICl; (D) EMIAc; (E) BMIBF4. Normalization was performed by setting the absorbance at 409 nm to 1 at 0 detergent concentration. Panel (F) shows representative absorbance spectra for myoglobin with: 0 mM BMIBF4 0 mM EBB (black), 0 mM BMIBF4 1.75 mM EBB (green), 56.3 mM BMIBF4 0 mM EBB (blue), 56.3 mM BMIBF4 1.75 mM EBB (red). All data in panels (A–E) are averages of three independent samples and error bars represent the standard deviation of the replicates.