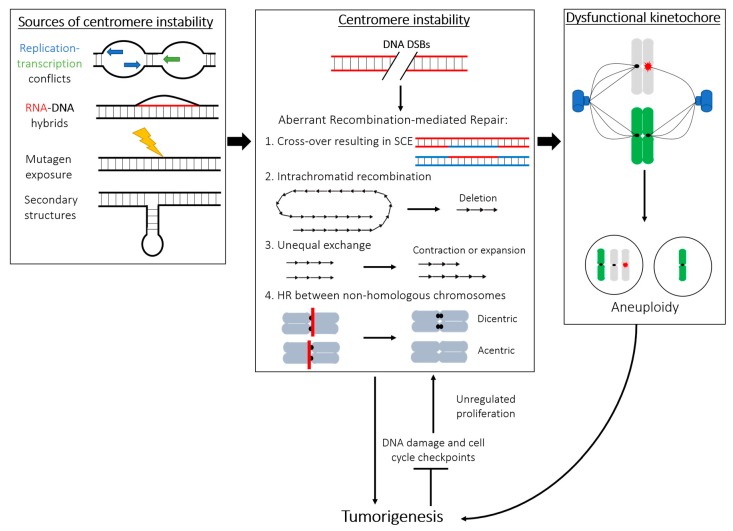
Figure 1.
Centromere DNA instability as a contributor to aneuploidy and carcinogenesis. Left panel. Sources of centromere instability, such as replication–transcription conflicts, RNA–DNA hybrids forming R-loops, mutagen exposure and stochastic damage, as well as various secondary structures may underlie the inherent fragility of centromeres and propensity for DNA damage. Middle panel. These burdens may result in DNA double-stranded breaks (DSBs) formed at the centromere. Repair of these DSBs through recombination-dependent pathways, such as homologous recombination (HR), may disrupt centromere integrity in several ways: (1) Crossover between sister chromatids will lead to sister chromatid exchange (SCE), which has been reported at human centromeres. (2) Search for the homologous sequence may erroneously identify an identical or nearly identical sequence within the same chromatid downstream or upstream of the break site. Recombination between these two regions would result in DNA excision into an extrachromosomal circle and subsequent loss of alpha-satellite sequences. (3) Recombination may lead to unequal exchange, thereby introducing instability in the total size of the centromeric array. (4) HR at identical centromere sequences between different chromosomes can lead to the formation of dicentric and acentric chromosomes. Right panel. Rearrangements, changes, and especially loss, of alpha-satellite DNA may impact the centromere and, in turn, affect the formation of a functional kinetochore during mitosis. Centromere/kinetochore dysfunction increases the risk of chromosome missegregation and formation of aneuploid daughter cells. Aneuploidy can, in turn, promote tumorigenesis. Other routes may exist by which centromere instability promotes malignant transformation independent of kinetochore establishment, including altered transcription, delayed decatenation, structural integrity, etc. (see text in Section 4). Canonical DNA damage and cell division checkpoints become activated at specific stages during this process to prevent transformation. Inhibition of these failsafe pathways that restrict unregulated proliferative growth and continued cycling with subsequent increase in centromere/genome instability causes a short circuit with deleterious consequences that further promote mutagenesis and cancer formation.