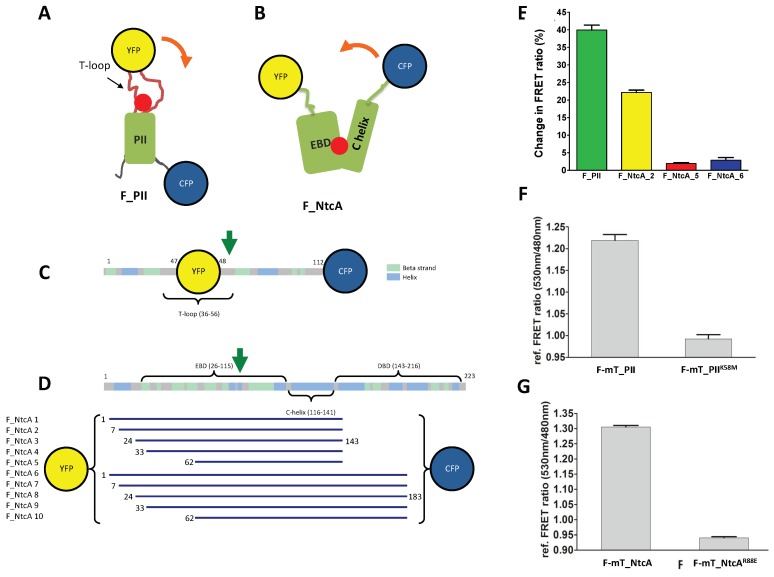
Figure 1.
2-oxoglutarate (2-OG) fluorescence resonance energy transfer (FRET) biosensors based on mono-proteins. (A,D) Schematic (A) and linear (C) representations of the FRET biosensor F_PII. The CFP is fused at the C-terminal end of PII whereas the YFP is inserted within the flexible, 2-OG-responsive T-loop; (B,D) schematic (B) and linear (D) representations of the FRET biosensor F_NtcA. The DNA binding domain was removed and the biosensors have conserved parts of the effector binding domain (EBD) and of the C-helix. The YFP is fused at the N-terminus of the EBD fragments whereas the CFP is fused to the C-terminus of the C-helix fragment. The yellow and blue spheres correspond to YFP and CFP, respectively. The red ball indicates the 2-OG binding site. The orange arrow indicates the conformational changes triggered by 2-OG binding. The green arrows indicate the substituted residues that prevent 2-OG binding and used as controls (K58M for PII, R88E for NtcA). The 10 different NtcA biosensor length variants, highlighting the first and last residues of each construct, are shown in panel (D); (E) FRET ratio change (%) of the biosensors purified after the addition of 1mM of 2-OG; (F,G) In vitro relative FRET ratio of F-mT_PII and its K58M mutant (F) and of F-mT_NtcA_2 and its R88E mutant (G) after addition of 10 mM of 2-OG (FRET ratio without 2-OG was set as 1).