Figure 9.
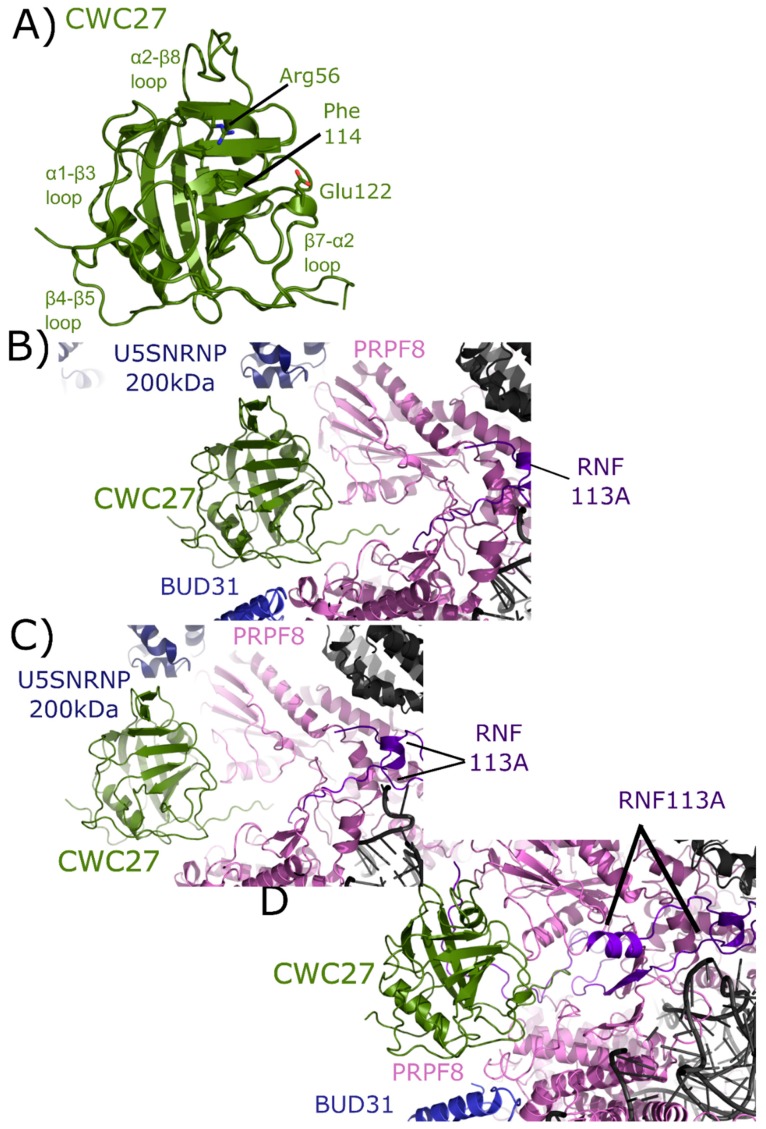
Structures of CWC27 in and out of the spliceosome. In (A) the isomerase domain of CWC27 is shown in cartoon representation (overlay of PDB IDs: 2HQ6 and 4R3E). Selected catalytic residues and protein–protein interaction regions are labeled. The substitution of Glu122 in the active site renders CWC27 inactive, although it still binds proline-containing peptides. In (B) the neighborhood around the isomerase domain of CWC27 in the mature Bact complex (PDB ID: 5Z56). Modeled interactions with U5 snRNP 200 kDa, BUD31, PRPF8, and RNF113 are highlighted. In (C), the neighborhood around CWC27 in the late Bact complex (PDB ID: 5Z58). The view is very similar to that in PDB ID: 5Z56, save for the absence of BUD31 and slight movement of PRPF8. In (D), the neighborhood around CWC27 in the Bact complex (PDB ID: 6FF4) is shown. Again, the modeled interactions are very similar to those in (B,C), with more of RNF113 modeled in 6FF4, including an additional, extensive interaction with β1–β2 of CWC27. The model includes BUD31 but U5 snRNP 200 kDa is missing. All models have only the isomerase domain of CWC27, with ≈200 additional residues uncharacterized.