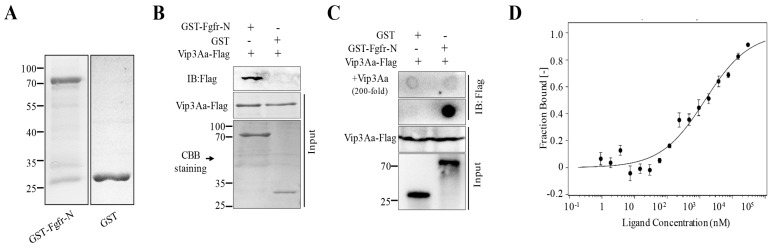
Figure 2.
The extracellular regions of Sf-FGFR could bind to Vip3Aa. (A) The purified GST-FGFR-N and GST protein. (B) The purified GST-FGFR-N and GST were mixed with Vip3Aa-Flag, respectively, and then the GST-Sepharose affinity beads were added followed by immunoblotting (IB) with the primary antibody (Mouse anti-Flag). (C) GST and GST-FGFR-N proteins were dotted on a PVDF membrane, respectively, and were incubated with Vip3Aa-flag (100 nM) or Vip3Aa-flag plus excess unlabeled Vip3Aa (200-fold), followed by immunoblotting with the primary antibody (Mouse anti-Flag). (D) The binding affinity of Vip3Aa with GST-FGFR-N was analyzed with MST. The labeled Vip3Aa was kept constant at 10 nM and the GST-FGFR-N was titrated from 0.3 nM to 10 µM. The equilibrium dissociation constant (Kd, mean ± SD) was the fitting result of three independent experiments.