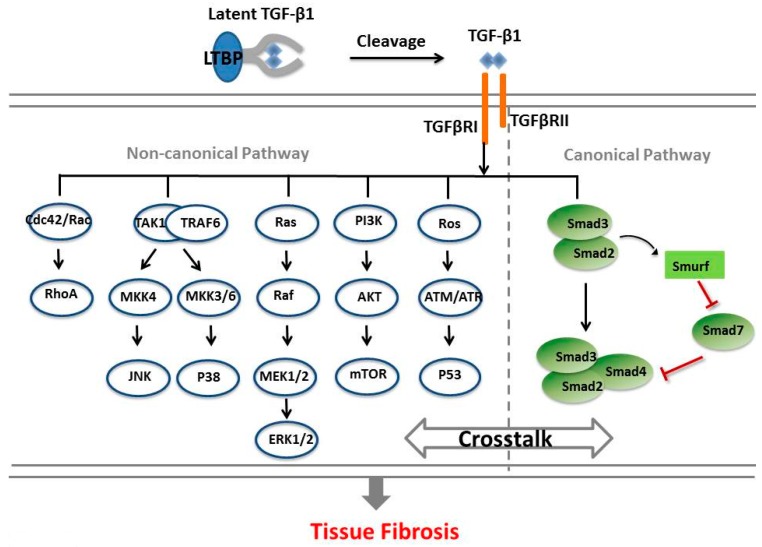
Figure 1.
Transforming growth factor-β 1 (TGF-β1) mediates a signaling pathway in tissue fibrosis. The latent TGF-β binding proteins (LTBP) complex is cleaved by proteases to release the active TGF-β1 that binds to the extracellular domain of TGF-β receptor type II (TβRII). The activated TβRII then phosphorylates TGF-β receptor type I (TβRI) kinase, thus triggering downstream signaling via either or both of the canonical (Smads-dependent) and noncanonical (Smads-independent) pathways. In the canonical pathway, TβRI phosphorylates Smad2 and Smad3, and then these Smads bind with Smad4 and this complex translocates into the nucleus. Meanwhile, TGF-β1 also activates Smad ubiquitin regulatory factor (Smurf) to degrade Smad7 to further enhance signaling. On the other hand, TGF-β1 can also induce profibrotic responses via a noncanonical pathway in a Smads-independent manner. TGF-β1 activates extracellular signal-regulated kinase (ERK) activation (Ras recruits Raf to the plasma membrane and leads to activation of ERK through mitogen-activated protein kinase (MEK)); c-Jun amino terminal kinase (JNK)/p38 activation JNK and p38 are at the tertiary layer of the mitogen-activated protein kinase (MAPK) pathway, in which they are activated by the MAP kinase kinases (MKKs), MKK4 and MKK3/6, respectively); Rho-like GTPases activation (the Rho-like GTPases include RhoA, Rac, and Cdc42); Phosphoinositide3-kinase/RAC-alpha serine/threonine-protein kinase (PI3K/AKT) activation (AKT is activated via PI3K, which then controls translational responses through mammalian target of rapamycin (mTOR)); induction of reactive oxygen species (ROS) (hypoxia-responsive element activity and hypoxia-inducible factor-1α expression by TGF-β1, then the p53 tumor suppressor can be induced). In addition, crosstalks may occur between TGF-β1/Smad and other pathways during tissue fibrosis.