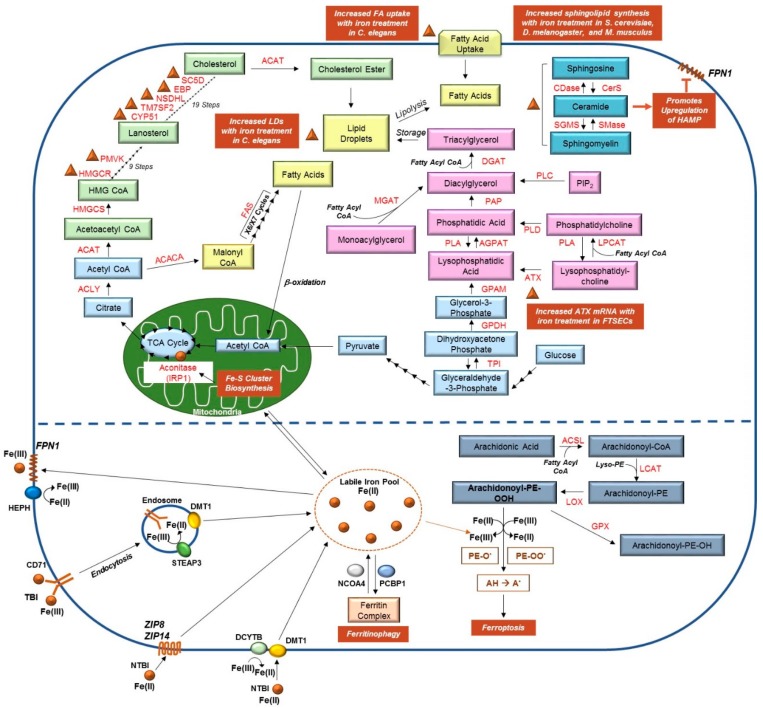
Figure 1.
Links between Lipid and Iron Metabolic Pathways. (Top) Pyruvate, a product of glycolysis, is converted to acetyl-CoA in the mitochondria. Acetyl-CoA feeds into the Krebs (TCA) cycle, illustrated in light blue, to generate citrate; the conversion of citrate to isocitrate is mediated by the enzyme aconitase and requires binding to Fe-S clusters (as indicated by the brown circle). Citrate can also be transported from the mitochondria to the cytosolic compartment where it is used to generate acetyl-CoA; this molecule can then feed into either the cholesterol biosynthetic pathway (green) or the fatty acid synthesis pathway (yellow). Elevated liver iron concentrations correlated with increased mRNA expression of several genes involved in cholesterol biosynthesis (namely, HMGCR, PMVK, CYP51, TM7SF2, NSDHL, EBP, and SC5D), as indicated with brown triangles (see Section 2.1). Exogenous fatty acids can also be imported into the cell; together, fatty acids, triacylglycerides, and cholesterol esters are essential components of lipid droplets. As detailed in Section 2.4, iron can promote both fatty acid import and lipid droplet formation. Synthesis of triacylglycerides, as well as phospholipids (and their modification), are presented in pink, whereas the sphingolipid metabolic pathway is displayed in dark blue. Iron can promote the production of ceramide (indicated with a brown triangle), and in turn induces HAMP expression, which negatively regulates FPN1 (refer to Section 2.2). Furthermore, our own unpublished work suggests iron treatment in human fallopian tube secretory epithelial cells (FTSECs) increases mRNA expression of autotaxin (ATX), which is involved in generating lysophosphatidic acid (see Section 2.5). (Bottom) TBI can be imported via endocytosis by binding to CD71; Fe(III) is then converted to Fe(II) by STEAP3 prior to being transported from the endosomal compartment to the cytosolic LIP by DMT1. Alternatively, NTBI can be imported into cells by DMT1 (following conversion of Fe(III) to Fe(II) by DCYTB), ZIP8, or ZIP14 [37]. From the LIP, iron may be (a) transported to the mitochondria for use in Fe-S cluster generation, (b) loaded to ferritin by PCBP1, or (c) used by the cell for other cellular processes. Iron can also be released from the ferritin complex via NCOA4-mediated ferritinophagy. Increased iron levels in the cell can promote the formation of lipid peroxides, a process critical for ferroptosis (shown in grey). Please see [2,38,39,40,41,42,43,44,45] for comprehensive reviews of these pathways and the contributing enzymes.