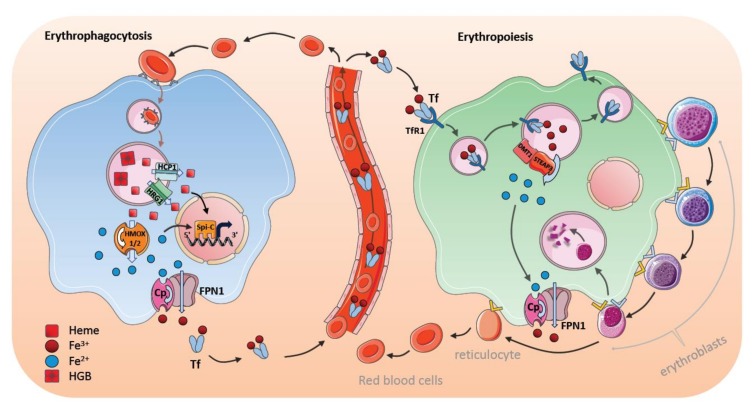
Figure 3.
Macrophages in erythrophagocytosis and erythropoiesis. The majority of iron in a macrophage is obtained during erythrophagocytosis. Splenic red pulp macrophages (RPMs) and liver Kupffer cells (KCs) is the main source of iron in the body. Senescent red blood cells (RBCs) are recognized by macrophages and digested in the lysosomes. Heme is extracted in the phagolysosomes and transferred to the cytosol by heme transporters heme-carrier protein 1 (HCP1) and heme responsive gene 1 protein (HRG1). Heme degradation by heme oxygenase 1 (HMOX1) in the cytosol provides ferrous iron that enters the intracellular labile iron pool. Iron is either stored in ferritin or exported by ferroportin 1 (FPN1) and ceruloplasmin (Cp). The transcription factor (Spi-C) protects iron processing RPMs in the spleen and BM nurse macrophages. Central nurse macrophages in the bone marrow (BM) promote erythropoiesis in the erythroblastic island niche. These macrophages ubiquitously express transferrin receptors and thus, probably mainly utilize transferrin (Tf)-bound iron. Nurse macrophages are important for the development of erythroblasts. This is mediated by adhesion molecules that control proliferation and differentiation of erythroblasts, enable erythroblast nuclei ingestion, and control the subsequent release of reticulocytes into the blood stream. Central macrophages are supposed to be involved in supplying maturing erythrocytes with iron to trigger heme synthesis.