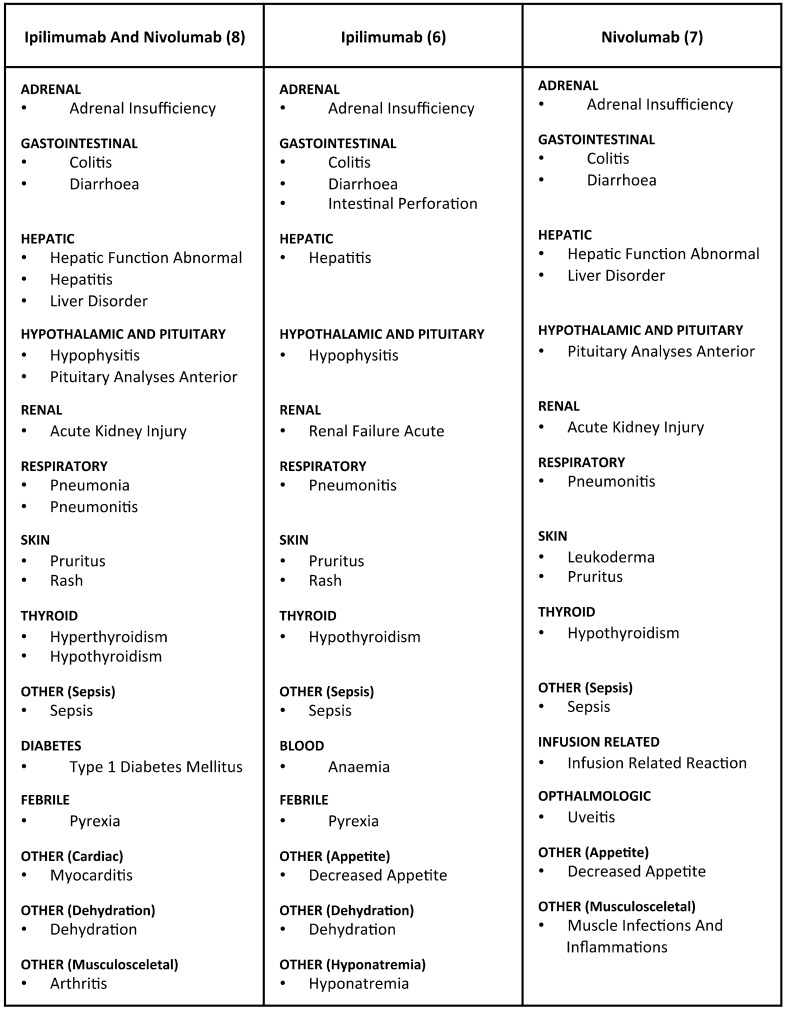
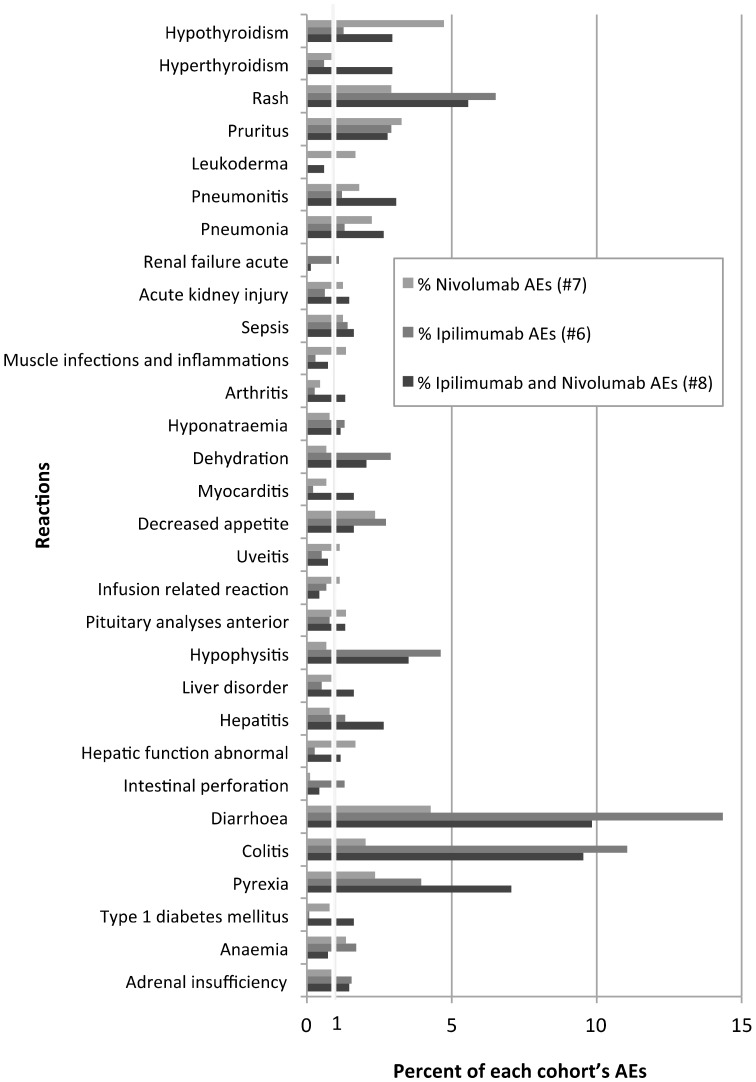
Figure 3.
The safety profiles of ipilimumab, nivolumab and their combination. The table (upper) lists the identified reactions organized by affected organ/system. For the selection, statistical significance (p-value < 0.05), PRR signal > 1, and occurrence in at least 1% of a cohort’s AEs (lower) were required. While some reactions were specific to one set, many were reported in all three, but with different signals. For example, ‘Hypophysitis’ was reported in 125 AEs of the ipilimumab cohort and in 24 AEs of the ipilimumab plus nivolumab cohort—when, in comparison, overall it was mentioned in a total of 475 AEs in FAERS—thus rendering it the reaction with the highest PRR signal for these cohorts (PRR#6: 1051; PRR#8: 621). Similarly, ‘Leukoderma’ had the strongest PRR signal among nivolumab reactions (PRR#7: 2439), reported in fifteen AEs of this cohort, when there were only seventy AEs in FAERS mentioning it altogether. Similarly, ‘Myocarditis’ (with 2713 AEs in FAERS) was reported in eleven AEs of the ipilimumab plus nivolumab combination cohort (PRR#8: 47.5), and ‘Colitis’ (with 12513 AEs in FAERS) was reported in 299, eighteen, and 65 AEs of the ipilimumab, nivolumab, and the ipilimumab plus nivolumab cohorts (PRR#6: 72; PRR#7: 12.9; PRR#8: 61), respectively.