Figure 5.
HSP90 Plays a Key Role in the Recovery of SUMO2/3 Modification following Heat Shock
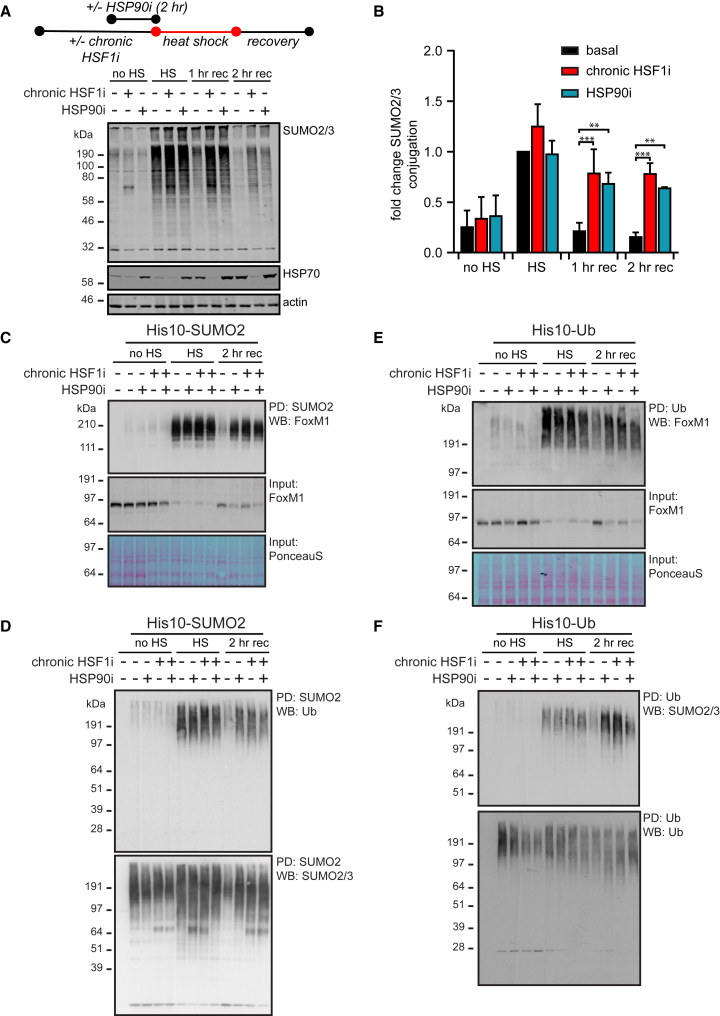
(A) HEK293T-REx cells expressing Dox-inducible dn-cHSF1 were either treated with Dox for 48 hr (chronic HSF1i) prior to heat shock (HS), with 500 nM STA-9090 (HSP90i) 2 hr prior to HS, or with a combination of both. Cells were exposed to a HS at 43°C for 75 min before returning to 37°C for a recovery period (rec) and lysed as indicated. Total amounts of SUMOylated proteins were analyzed by immunoblotting. HSP70 levels were used to confirm induction of the heat shock response and functional HSF1 inhibition.
(B) Quantification of fold change of SUMOylation from (A) as compared to SUMOylation after HS under the basal proteostasis conditions. n = 3, error bars indicate SD. Significance was determined using ANOVA analysis followed by post hoc Tukey analysis, ∗p < 0.05; ∗∗∗p < 0.0005.
(C) HEK293T-REx cells stably co-expressing His10-SUMO2 and Dox-inducible dn-cHSF1 were treated as in (A) with a 2-hr recovery at 37°C. SUMOylated proteins were purified by means of His10-purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
(D) As for (C). Elutions were analyzed by immunoblotting for Ub and SUMO2/3.
(E) HEK293T-REx cells stably co-expressing His10-Ub and Dox-inducible dn-cHSF1 were treated as in (A) with a 2-hr recovery at 37°C. Ubiquitinated proteins were purified by means of His10-purification. Elutions and inputs were analyzed by immunoblotting for FoxM1. Ponceau S stain was used as a loading control for inputs.
(F) As for (E). Elutions were analyzed by immunoblotting for SUMO2/3 and Ub.
See also Figure S6.