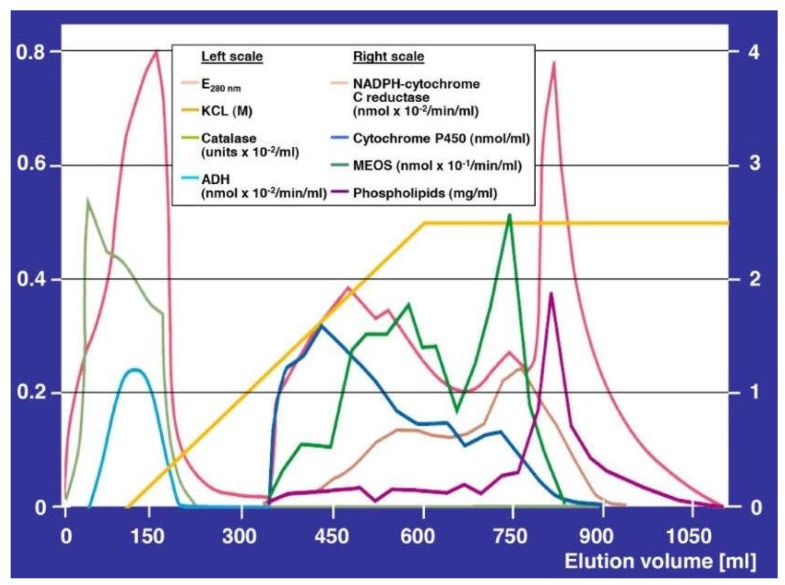
Figure 2.
Purification of the microsomal ethanol-oxidizing system (MEOS) and its separation from catalase and alcohol dehydrogenase (ADH) activities. Separation was achieved by DEAE (Diethyl-Amino-Ethyl) cellulose ion exchange column chromatography after solubilization of liver microsomes obtained from rats fed an ethanol containing liquid diet for three weeks. In the void volume eluted up to around 220 mL, the highest peak represents the protein curve assessed as E280 nm, and the peak below that is the catalase peak, whereas ADH presents as the lowest peak. Starting with an elution volume of around 330 mL, microsomal components begin to appear. The first peak represents cytochrome P450, the second peak represents E280 nm, followed by a third peak with two shoulders and by a fourth peak representing MEOS. At around 770 mL, the reductase peak emerges, followed by the phospholipid peak at around 790 mL elution volume. Overall, this experimental approach was challenging, putting active MEOS on the top of the column and expecting active MEOS in the effluents. There was a high risk of inactivation of MEOS, not only during the solubilization procedure using ultrasonication and deoxycholate that disintegrated MEOS out of the intact microsomal membranes, but also during the chromatography procedure itself that could lead to the inactive cytochrome P420 from the active P450. The original figure was published in a previous report [30] and is reproduced with permission of the Publisher Elsevier (Amsterdam, The Netherlands).