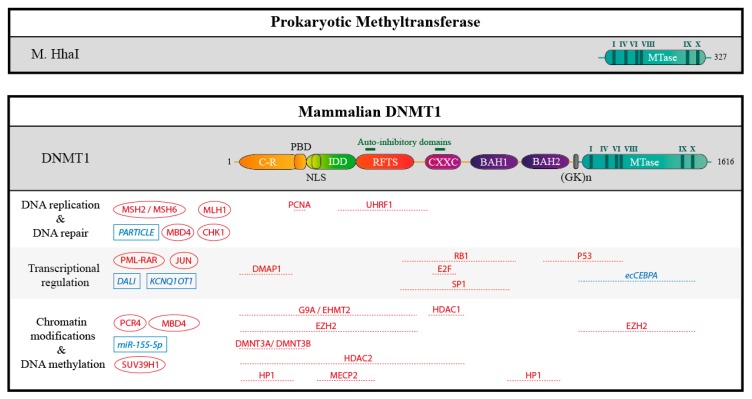
Figure 4.
Schematic structure of the prokaryotic methyltransferase M. HhaI, compare to DNMT1 and partners. The human DNMT1 contains 1616 amino acids residues. The catalytic methyltransferase domain (MTase, in blue) is very similar to that of the prokaryotic methyltransferase M. HhaI and harbors highly conserved motifs (I-X, in dark blue). In addition, DNMT1 harbors a charge-rich (C-R) domain containing the proliferating cell nuclear antigen (PCNA) binding domain (PBD), an intrinsically disordered domain (IDD) with a nuclear localization sequence (NLS), a replication foci target sequence (RFTS), a zinc finger domain (CXXC), and two bromo-adjacent homology domains (BAH 1/2). The catalytic and the regulatory domains are connected by a series of Gly-Lys repeats. Auto-inhibitory domains are highlighted in green. In addition, some interacting proteins and RNAs are represented: if they are known, mapped interaction domains are indicated. Partners with unknown binding sites are shown on the left. Proteins are depicted in red, RNAs in blue.