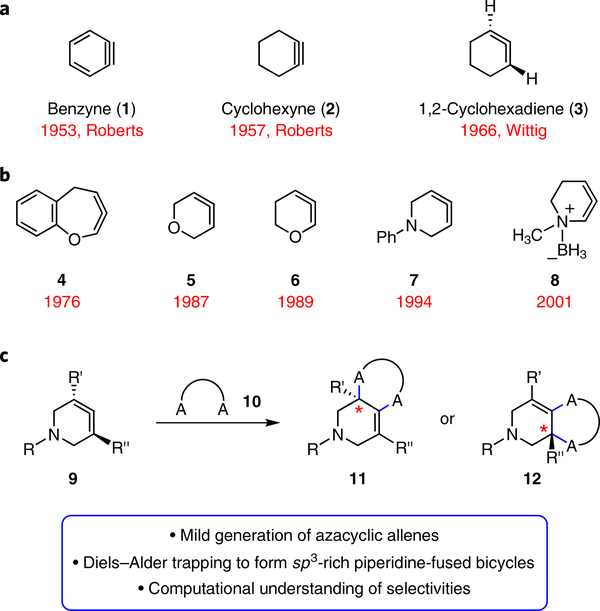
Fig. 1 |. Survey of strained cyclic intermediates and focus of present study.
a, Cyclic alkynes such as benzyne (1) and cyclohexyne (2) were once avoided, but have now been used in many synthetic applications, including ligand synthesis, natural product synthesis, agrochemistry and materials science. 1,2-Cyclohexadiene (3) has been studied computationally and evaluated in cycloaddition reactions since its discovery in 1966, but is underutilized in chemical synthesis. b, Heterocyclic allenes are even less utilized in synthetic applications. Seminal examples of previously generated heterocyclic allenes include compounds 4 –8. c, The present study focuses on the generation and trapping of azacyclic allenes (9) in Diels–Alder and other cycloadditions to furnish products 11 or 12, where A is a carbon, oxygen or nitrogen atom.