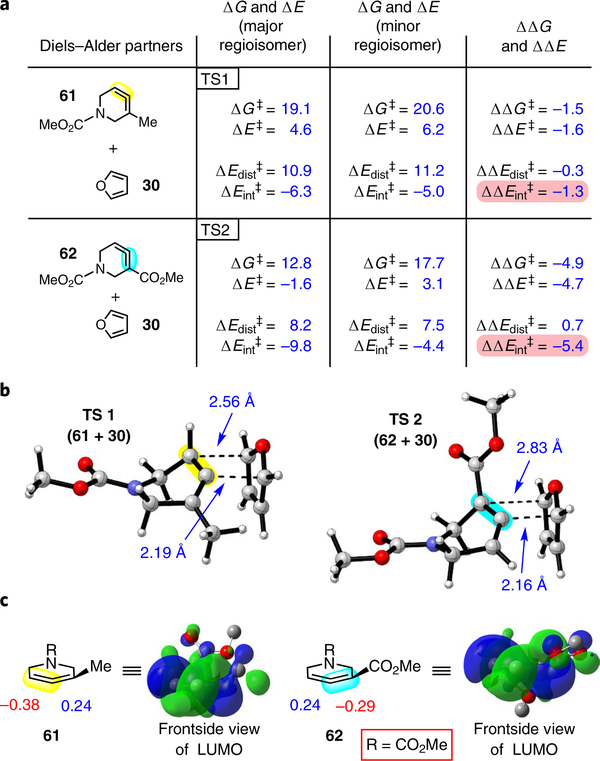
Fig. 4 |. Computations provide insight into regio- and diastereoselectivities.
a, Reaction of furan (30) with allenes 61 and 62 to give endo products was examined computationally (ω B97XD/6–311+ G(d,p)/SMD(MeCN)). The ΔG‡ and ΔE‡ values determined were consistent with experimental regiochemical observations. A distortion/ interaction analysis was performed, where the ΔE‡ was broken down into the ΔE‡ of distortion and the ΔE‡ of interaction. The ΔE‡ of interaction was found to be a significant contributor to the difference in ΔE‡ values for both allenes 61 and 62. b, Optimized endo transition states leading to the observed major regioisomers (ωB97XD/6–31G(d)). c, Orbital coefficients for 61 and 62 and depiction of LUMOs (HF/6–31G). Highlighted bonds indicate which double bond of the cyclic allene undergoes cycloaddition, with colours corresponding to the methyl and ester group substituents depicted in Fig. 3. Pink shading emphasizes that the magnitude of the ΔΔE‡ of interaction is significant and leads to the observed regiochemistry.