Table 1.
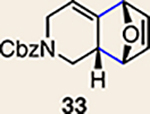
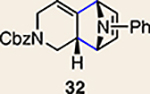
Scope of Diels-Alder cycloadditions of azacyclic aliene intermediates 25–28
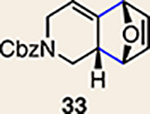
| Entry | Allene | Diene | Products (yield, d.r.) | Entry | Allene | Diene | Products (yield, d.r.) |
|---|---|---|---|---|---|---|---|
| 1 | 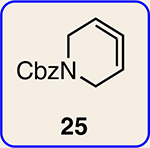 |
 |
 |
7 | 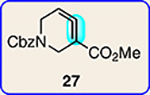 |
 |
 |
| 2 | 25 |  |
 |
8 | 27 | 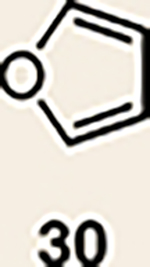 |
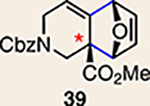 |
| 3 | 25 | 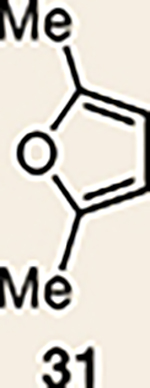 |
 |
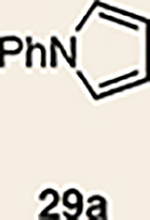
9 | 27 | 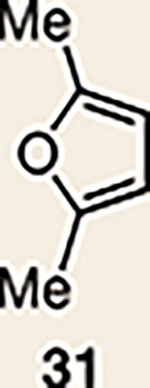 |
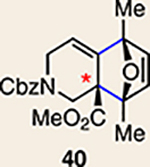 |
| 4 | 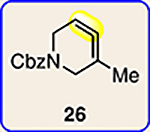 |
 |
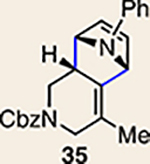 |
10 | 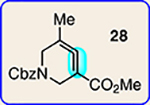 |
 |
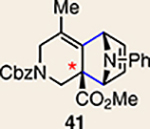 |
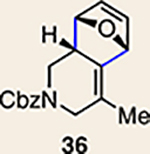
| 5 | 26 | 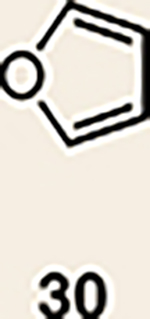 |
 |
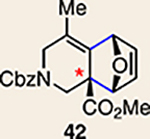
11 | 28 | 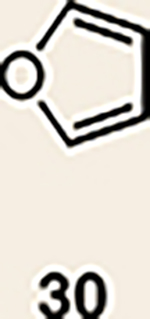 |
 |
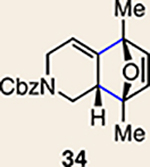
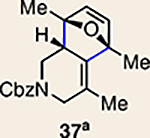
| 6 | 26 | 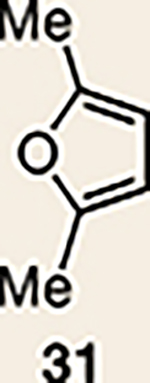 |
 |
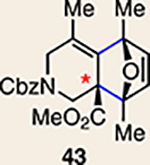
12 | 28 | 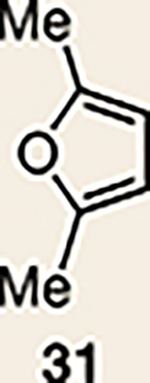 |
 |
Conditions unless otherwise stated: silyl triflate substrate (1.0equiv.), diene (5.0–10.0equiv.), CsF (5.0equiv.), acetonitrile (0.1 M) at 23°C. Yields shown reflect the average of two isolation experiments.
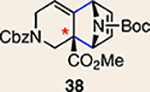
The regioisomer of 37 was also observed (∼20% yield), resulting from endo cycloaddition on the more substituted olefin of allene 26.
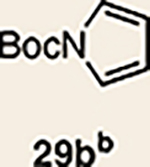
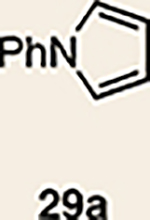
Pyrrole 29b was used in place of pyrrole 29a, as the cycloaddition of 29a with 27 proceeded in low yield for reasons that are not presently understood. Highlighted bonds indicate which double bond of the cyclic allene undergoes cycloaddition, with colours corresponding to the methyl and ester group substituents depicted in Fig. 3. Cbz, carboxybenzyl; A, methyl group or hydrogen atom; X, oxygen atom, NBoc or NPh; Boc, ferf-butyloxy carbonyl.
