Hospital use and charges were reduced by a caregiver coaching intervention used to support care of patient crises and discharge transitions.
Abstract
OBJECTIVES:
We sought to examine the effect of a caregiver coaching intervention, Plans for Action and Care Transitions (PACT), on hospital use among children with medical complexity (CMC) within a complex care medical home at an urban tertiary medical center.
METHODS:
PACT was an 18-month caregiver coaching intervention designed to influence key drivers of hospitalizations: (1) recognizing critical symptoms and conducting crisis plans and (2) supporting comprehensive hospital transitions. Usual care was within a complex care medical home. Primary outcomes included hospitalizations and 30-day readmissions. Secondary outcomes included total charges and mortality. Intervention effects were examined with bivariate and multivariate analyses.
RESULTS:
From December 2014 to September 2016, 147 English- and Spanish-speaking CMC <18 years old and their caregivers were randomly assigned to PACT (n = 77) or usual care (n = 70). Most patients were Hispanic, Spanish-speaking, and publicly insured. Although in unadjusted intent-to-treat analyses, only charges were significantly reduced, both hospitalizations and charges were lower in adjusted analyses. Hospitalization rates (per 100 child-years) were 81 for PACT vs 101 for usual care (adjusted incident rate ratio: 0.61 [95% confidence interval 0.38–0.97]). Adjusted mean charges per patient were $14 206 lower in PACT. There were 0 deaths in PACT vs 4 in usual care (log-rank P = .04).
CONCLUSIONS:
Among CMC within a complex care program, a health coaching intervention designed to identify, prevent, and manage patient-specific crises and postdischarge transitions appears to lower hospitalizations and charges. Future research should confirm findings in broader populations and care models.
What’s Known on This Subject:
Hospital use, some of which may be preventable, comprises a disproportionate share of health care spending for children with medical complexity. Although complex care programs seek to reduce hospitalizations, feasible evidence-based interventions are limited.
What This Study Adds:
This caregiver coaching intervention designed to manage patient crises and hospital-to-home transitions was used to reduce hospitalizations and charges among low-income children with medical complexity receiving care within a complex care medical home.
Complex care programs seek to influence key health outcomes for children with medical complexity (CMC), and investment in program infrastructure is often justified by anticipating savings from lower health care use. Because hospital care accounts for ˃50% of spending for CMC,1,2 avoiding hospitalizations has become a major focus. Although some hospital use is inevitable for children with unpredictable and fragile underlying conditions, growing evidence suggests that reducing hospitalizations for CMC is achievable.3,4
One randomized controlled trial (RCT) revealed nearly 50% reduction in hospitalizations for CMC enrolled in a complex care medical home.5 Authors of observational studies have found lower CMC–hospital use with care coordination, care planning, and home visitation.6–10 Authors of recent studies among adults with chronic illness link avoidable hospital use to caregiver knowledge, skill, and confidence to manage health.11,12 We have observed that higher parent confidence predicts fewer hospitalizations 1 month after discharge.13 Research designed to test theoretically grounded interventions is needed now to help families, providers, researchers, and policymakers more efficiently invest resources.
We previously developed a key driver framework for reducing hospitalizations for CMC.14 A caregiver coaching model (ie, Plans for Action and Care Transitions [PACT]) was conceived to prevent hospitalizations for CMC by influencing the following 2 key drivers: (1) early recognition of critical symptoms and execution of crisis plans and (2) seamless, comprehensive hospital transitions.14 Our objective was to test in an RCT the effectiveness of PACT to reduce hospitalizations for CMC within a complex care medical home.
Methods
We included all CMC <18 years of age enrolled in the Pediatric Medical Home Program at the University of California, Los Angeles (UCLA). Children are eligible for this program if they live in Los Angeles County and qualify for at least 2 specialty care centers through California Children’s Services, California’s Title V program for low-income children with special health care needs.
Enrollment occurred in person or by phone between December 2014 and January 2015. Participants remained enrolled for up to 18 months, with the intervention ending September 30, 2016. The trial was prospectively designed to last 18 months and had no interim stoppage rules. Patients ≥18 years old and those whose caregivers were neither English- nor Spanish-speaking were excluded.
Participants were randomly assigned to intervention or usual care in a 1:1 ratio by using random permuted blocks of 2 or 4 and stratified by caregiver-identified primary language (English or Spanish) and past use (being in the top 20 percentile or not for total number of emergency department [ED] visits plus hospitalizations at UCLA in the year before randomization). Randomization was computer generated by a biostatistician who had no patient involvement. After participant consent, research assistants blinded to treatment allocation called the project manager (A.A.S.), who held the blinded treatment allocation sequences, to learn which arm the participant was assigned. Eligible siblings were allocated to the same treatment arm, but only 1 randomly selected sibling was enrolled for data collection.
Usual Care: Pediatric Medical Home Program at UCLA
Established in 2003, the Pediatric Medical Home Program at UCLA is used to deliver primary care, urgent care, and care coordination services to CMC.8 The clinical team is composed of general pediatricians, a pediatric nurse practitioner, and 3 bilingual care coordinators. Patients receive extended visits, comprehensive care planning, subspecialty comanagement, case management, and communication with community services. Patients are typically seen every 3 to 6 months for care-plan updates, well-child checks, and as needed for urgent care. Previous analyses have revealed a pre-post 50% reduction in ED visits in the year after enrollment8 and high family satisfaction with the program.15
Intervention: PACT
Using a structured approach over a 12-month period, we developed the PACT intervention by integrating findings from systematic literature review,3 in-depth caregiver interviews,16 and the RAND-UCLA Appropriateness Method with a national expert panel,14 each focused on preventing the hospitalization of CMC. The Medical Home Program clinical and research teams collaborated in all aspects of intervention development, implementation, and testing. Before the trial, the program’s parent advisory group provided feedback on development and implementation at regular monthly meetings. Caregivers of 19 children were involved in the advisory group during this period, 13 of whom enrolled in the study (9 allocated to PACT and 4 to usual care).
PACT has the following 2 elements: (1) customized written plans used to identify and address patient- and family-centered triggers of hospital use (“action plans”) and (2) care transition coaching around hospital discharge.
Action plans for intervention patients were created in caregivers’ preferred language by a medical home physician or nurse practitioner via a systematic protocol. The format was adapted from asthma action planning.17 In the first step, areas of focus were identified on the basis of the patient’s history and caregiver opinion about what was most likely to lead to a future hospitalization (eg, seizures, breathing difficulty). In the second step, objective and subjective signs of baseline (green), worsening (yellow), and severe (red) statuses were defined (eg, seizures occurring more than once per hour). In the third step, the specific actions caregivers should take to prevent or manage each status were delineated. At times of crisis, depending on circumstances, the plan may direct families to call their primary care provider, specialist, or go directly to the ED. The goal of action planning was to provide families with confidence and direction to manage crises in the most appropriate setting. Action plans were developed and refined at scheduled outpatient program visits and after unscheduled hospitalizations. Patients could have ˃1 action plan. Explicit caregiver input played an integral role in each stage of action plan development and refinement, and teach-back was used after the plan was created. Lastly, each action plan was discussed at weekly Medical Home Program meetings for feedback and continued improvement (eg, Was the plan clear and sufficiently detailed? Were any updates needed?).
Care transition support was accomplished by adapting the care transitions intervention (CTI), developed by Coleman et al,18 for the pediatric population. The CTI is built on the following 4 conceptual pillars: (1) medication self-management, (2) patient-centered record owned and maintained by the patient to facilitate cross-site information transfer, (3) timely follow-up with primary or specialty care, and (4) a list of “red flags” and instructions on how to respond to them. Transition coaches facilitate intervention activities, including meeting patients before discharge, conducting home visits within 72 hours postdischarge, and conducting 3 phone calls within 30 days postdischarge. During structured home visits, the health coach reviewed each of the 4 pillars with the family, elicited caregiving goals, and focused coaching activities on needs identified by families. Phone calls were used to discuss caregiver progress toward their goals and again to review the 4 pillars. One full-time Spanish and English bilingual individual with a bachelor’s degree and previous health coaching experience was hired to deliver the CTI to intervention patients. This nonclinical coach was trained to implement the CTI through the Care Transitions Program in Colorado, participating in monthly community learning calls with peer transition coaches, conducting mock visits, and receiving feedback from clinician-observed visits.
Outcomes
The primary study outcomes were number of hospitalizations and readmissions. UCLA hospitalizations were identified through administrative records; those outside UCLA were reported by caregivers every 3 months. We did not exclude any specific hospitalization types because we hypothesized that elective or “planned” hospitalizations may still represent potentially preventable visits (eg, improved care might lead to bundling multiple elective procedures into a single stay). Readmissions were all-cause UCLA hospitalizations occurring within 30 days from discharge. No patients had oncologic, hemodialysis, or inpatient rehabilitative scheduled readmissions. Secondary outcomes were total UCLA charges (preplanned secondary outcome) and death (a post hoc secondary outcome). Charges included all professional, facility, laboratory, radiology, and pharmacy charges from inpatient and outpatient settings (minus outpatient pharmacy charges, which were unavailable).
Patient Characteristics
Patient characteristics included demographics, duration in the Medical Home Program, number of visits in the year before randomization (Medical Home Program, hospitalizations, and ED), and complex chronic conditions (CCCs) and technology assistance as defined by Feudtner et al.19 Caregiver characteristics included primary language, income, highest education, and family size.
Statistical Analysis
All analyses followed the intent-to-treat principle to avoid bias from selective intervention dropout. Differences in baseline characteristics between PACT and usual care groups were assessed with t tests or Wilcoxon rank tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables. Negative binomial regression with robust SEs were used to estimate incident rate ratios of primary outcomes. Stata’s (Stata Corp, College Station, TX) exposure variable option with study duration was used to account for the opportunity for outcomes to occur. In addition, Cox proportional hazards regression models with robust SEs were used to identify hazard ratios for experiencing a 30-day readmission. Kaplan-Meier estimates were used to assess readmission and mortality risk for treatment and usual care groups. Time was defined as the number of days from study enrollment until death or censoring. Patients who withdrew were considered as not meeting the primary end point and were censored at the time of withdrawal. Log-rank tests were used to identify a difference in Kaplan-Meier curves between intervention and usual care.
Charges were analyzed by using generalized linear models with a γ distribution and log link function after conducting a modified Park test.20 We expressed results as the mean charges (with SEs) for treatment groups and the mean difference in US dollars between PACT and usual care by using the postestimation predictive margins commands in Stata.
We hypothesized a priori that, with this small sample, treatment effects may be confounded by important constructs. Our conceptual model was informed by previous research revealing associations between hospital use (including readmissions) and demographics, medical complexity, severity of illness, and past use.21,22 We therefore planned to include prespecified and conceptually grounded covariates in multivariate regression models.23 We included both randomization stratification variables (language and past hospital plus ED use), CCCs (above or below median number), and the number of program visits 12 months before randomization (medical home engagement).
We planned to enroll 260 patients to identify a 50% reduction in hospitalizations between intervention and usual care groups (error of α .05; power level of 80%). At the time of these calculations, however, we overestimated the program’s future growth rate and had a smaller pool of eligible participants than expected. Analyses were performed by using Stata version 14.0. Statistical significance was concluded at the 2-sided significance level of 0.05. The study was approved by the UCLA Institutional Review Board.
Results
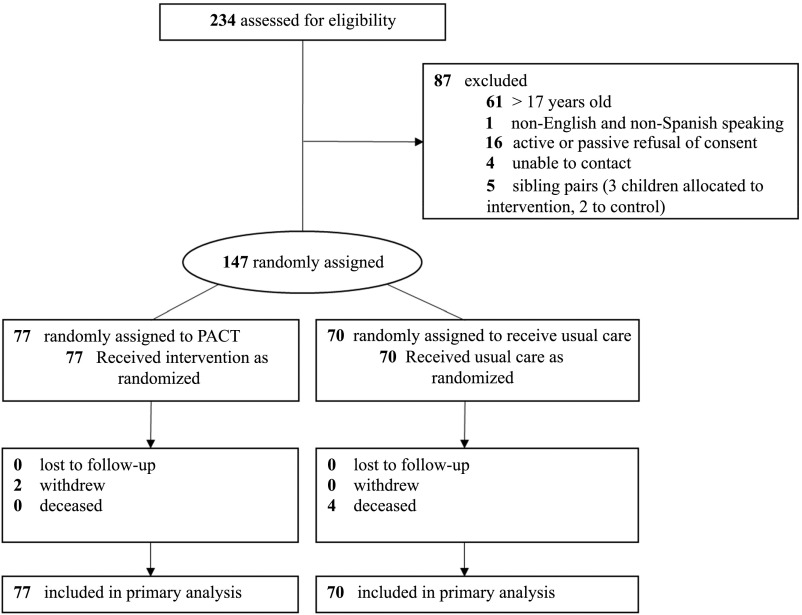
Of 167 eligible participants, 147 were enrolled and randomly assigned; 77 were assigned to the intervention group and 70 were assigned to usual care (Fig 1). Two intervention patients withdrew from the study (after 5 months and after 8 months) and 4 usual care patients died during the study. The average enrollment duration was 17.6 months for PACT and 17.2 months for usual care.
FIGURE 1.
Treatment assignment for CMC.
There were no significant differences in baseline characteristics between treatment groups (Table 1). Most patients were Hispanic, 56% were primarily Spanish-speaking, and 99% were publicly insured. At enrollment, mean child age was 8 years and mean duration in the Medical Home Program was 4 years. Children had 3.7 CCCs (SD 1.8), and two-thirds were technology assisted. The most common CCCs were gastrointestinal (62%), neuromuscular (57%), and cardiovascular (40%). In the year before randomization, children had a median of 1 hospitalization (interquartile range [IQR]: 0–2).
TABLE 1.
Baseline Characteristics by Treatment Group
| Treatment Group, No. (%) | ||
|---|---|---|
| PACT Intervention, n = 77 | Usual Care, n = 70 | |
| Age ranges, y | ||
| <6 | 23 (30) | 22 (31) |
| 6–8 | 22 (29) | 13 (19) |
| 9–11 | 17 (22) | 13 (19) |
| >11 | 15 (20) | 22 (31) |
| Female sex | 34 (44) | 26 (37) |
| Race and/or ethnicity | ||
| White, non-Hispanic | 9 (12) | 11 (16) |
| African American, non-Hispanic | 5 (7) | 3 (4) |
| Hispanic | 60 (78) | 50 (71) |
| Asian American and Pacific Islander | 1 (1) | 5 (7) |
| Other | 2 (3) | 1 (1) |
| Primary household language | ||
| English | 34 (44) | 31 (44) |
| Spanish | 43 (56) | 39 (56) |
| Public insurance | 76 (99) | 69 (99) |
| Primary caregiver education | ||
| Associate, college, or graduate degree | 11 (14) | 16 (23) |
| Some college, no degree | 16 (21) | 9 (13) |
| Completed high school, GED, or vocational program | 15 (20) | 19 (27) |
| High school, no diploma | 14 (18) | 16 (23) |
| Eighth grade or less | 21 (27) | 10 (14) |
| Family size | ||
| No. children in household, mean (SD) | 2.3 (1.2) | 2.2 (0.9) |
| Total No. household inhabitants, mean (SD) | 4.6 (1.6) | 4.5 (1.6) |
| Income | ||
| <$35 000 | 15 (20) | 19 (27) |
| Duration in medical home program, y, mean (SD) | 4.3 (3.1) | 3.8 (3.2) |
| Medical home visits, y before randomization, mean (SD) | 5.5 (4.1) | 5.2 (3.5) |
| CCCs,a mean (SD) | 3.5 (1.6) | 3.8 (2.0) |
| CCCsa | ||
| ≤3 CCC | 39 (51) | 36 (51) |
| >3 CCC | 38 (49) | 34 (49) |
| Technology assistanceb | 50 (65) | 49 (70) |
| ED visits, y before randomization, median (IQR) | 1 (0–3) | 1 (0–3) |
| Hospitalizations, y before randomization, median (IQR) | 1 (0–2) | 1 (0–2) |
PACT Intervention Experience
All patients received action plans, and 80% received 1 within 38 days from study enrollment. Over two-thirds of action plans were revised at least once during the study period, and 13 patients had multiple different action plans (Table 2). The 5 most common action plan focus areas were respiratory distress, fever, enteral feeding tube issues, general access (eg, how to reach needed care and/or providers outside of the ED at any hour), and seizures. With respect to care transitions, among intervention patients experiencing hospitalizations during the study period, 79% of expected home visits and over two-thirds of expected phone calls were successfully conducted. Home visits occurred within a median of 6 days postdischarge (IQR: 4–10).
TABLE 2.
Intervention Implementation Measures
| No. (%) | |
|---|---|
| Action plans | |
| Have at least 1 action plan | 77 (100) |
| >1 action plan | 13 (17) |
| Action plan revised during study | 53 (69) |
| Use of action plan in past 3 moa | 186 (40) |
| Most common action plan focus areas | |
| Respiratory distress or asthma | 28 (36) |
| Fever | 13 (17) |
| Enteral tube issues | 11 (14) |
| General accessb | 9 (12) |
| Seizures | 8 (10) |
| Care transitions | |
| Home visits completedc | 73 (79) |
| Phone calls completedd | 186 (67) |
| Visit time, min, mean (SD) | 47 (18) |
| Visit distance, miles, mean (SD) | 26 (35) |
Caregiver report that an action plan was used in the past 3 mo. Caregivers were asked every 3 mo during 18-mo study period (77 caregivers asked 6 times or 462 total queries).
General access action plans were focused on how to reach providers and care outside the ED (eg, on-call providers and evening and/or weekend clinics).
Denominator was 92 identified hospitalizations during the study period.
Denominator was 276 among 92 identified hospitalizations during the study period.
Caregivers were asked about intervention experience every 3 months with open- and closed-ended questions. Quotes were recorded verbatim. More than 40% of the time, caregivers reported needing to use their child’s action plan in the previous 3 months. Two representative quotes of caregiver action plan impressions included the following: “Parents ‘panic’ and forget who to contact or what to do, so the action plan helps by providing contact numbers, people to contact...I think it is useful in times when someone else is caring for her, like her home care nurse, so that this way, they know what to do” and, “I was happy to know what to do, because before having this, I wouldn’t know what to do and would sort of freak out. Her G-tube fell out, but this time, I knew what steps to take.” Over 88% of caregivers who had a home visit with the transition coach rated it “useful.” Two representative caregiver quotes about transition coaching included the following: “I like that when she came we made a plan and a list of things I had to do - I wrote down my questions for the doctors so that I wouldn’t forget” and, “It’s great to have someone help me follow up on her care - it helps a lot to have these home visits since I’m the only one who cares for her.”
Outcomes After PACT Intervention
Hospitalization rates were 81 per 100 child-years in PACT and 101 per 100 child-years in usual care (Tables 3 and 4). In adjusted analyses, significantly lower hospitalization incident rate ratios were observed in PACT versus usual care (adjusted incident rate ratio: 0.61; 95% confidence interval [CI] 0.38–0.97). Rates of all-cause 30-day readmissions were reduced in PACT (adjusted incident rate ratio: 0.37; 95% CI 0.14–0.98). Risk of experiencing a 30-day readmission after the first hospitalization was lower for children enrolled in PACT (adjusted hazard ratio: 0.41; 95% CI 0.17–1.00; Table 5; Supplemental Fig 2). Unadjusted mean charges were $33 645 in PACT and $59 604 in usual care (mean reduction: $25 960; 95% CI $5–$51 914). Adjusted mean charges were $14 206 lower (Table 4). Aggregate charges during the study were $944 759 lower for the 77 PACT participants than for the 70 usual care participants. The only deaths that occurred during the study period were in the usual care group (Kaplan-Meier estimated log-rank P = .04).
TABLE 3.
Outcome Measures by Treatment Group: Primary Outcomes
| Outcome | Intervention (n = 77) | Usual Care (n = 70) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| Rate per 100 Child-ya | Rate per 100 Child-yb | IRRc (95% CI) | P | IRRd (95% CI) | P | |
| Hospitalizations | 81 | 101 | 0.76 (0.41–1.39) | .37 | 0.61 (0.38–0.97) | .04 |
| Readmissions | 17 | 23 | 0.63 (0.19–2.06) | .45 | 0.37 (0.14–0.98) | .05 |
IRR, incident rate ratio.
Intervention subjects had 113.0 child-y.
Usual care subjects had 100.3 child-y.
Calculated from negative binomial regression models, enrollment duration modeled as exposure.
Models were adjusted for English or Spanish language, being in the top 20 percentile for the total number of ED visits and hospitalizations in the y before randomization, having above or below the median number of CCCs, and the number of medical home program visits in the y before randomization.
TABLE 4.
Outcome Measures by Treatment Group: Secondary Outcomes
| Intervention | Usual Care | Difference (95% CI) | P | |
|---|---|---|---|---|
| Chargesa, $, mean (SE) | ||||
| Unadjusted | 33 645 (5370) | 59 604 (12 105) | 25 960 (5–51 914) | .02 |
| Adjustedb | 38 155 (5649) | 52 361 (7868) | 14 206 (3386–31 789) | .02 |
| No. deaths | 0 | 4 | c | .04d |
Mean charges per child with SE for intervention and usual care groups during the study period were estimated from generalized linear models.
Models were adjusted for English or Spanish language, being in the top 20 percentile for the total number of ED visits and hospitalizations in the y before randomization, having above or below the median number of CCCs, and the number of medical home program visits in the y before randomization.
Not estimated because there were no events in the intervention group.
Determined from log-rank test.
TABLE 5.
Cox Proportional Hazards Risk of All-Cause 30-Day Readmissions or Mortality by Treatment Group
| Outcome | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Readmission | 0.46 (0.18–1.14) | .09 | 0.41 (0.17–1.00) | .05 |
| Death | N/Ab | <.001 | N/Ab | <.001 |
N/A, not applicable.
Models were adjusted for English or Spanish language, being in the top 20 percentile for the total number of ED visits and hospitalizations in the y before randomization, having above or below the median number of CCCs, and the number of medical home program visits in the y before randomization.
Hazard ratio was not estimated because there were no events in the intervention arm.
Discussion
Our main finding with this study was that a health coaching intervention designed to identify, prevent, and manage both patient-specific crises and the transition home after discharge reduced hospital use for CMC already enrolled in a mature complex care program. We also observed that those enrolled in PACT had fewer all-cause 30-day readmissions and were less likely to experience at least 1 readmission.
Although reducing hospitalizations is a goal of pediatric complex care programs7,24,25 (and 1 previous RCT reveals such an effect5) this is the first RCT used to investigate whether additional reductions in hospital use are possible through targeted enhancements within an existing complex care program. This research can be used to help identify the complex care processes that may be most effective at lowering hospitalizations.
The components of PACT were constructed from key drivers of CMC–hospital use3,14 and informed by experience from other populations. CTI has a well-established record for preventing adult hospitalizations as far as 3 months after discharge.18 It has been implemented in diverse settings26,27 and with family caregivers.28 This is the first study suggesting CTI effects may extend to CMC. These findings are aligned with those from several recent studies. For example, authors of a single-center study concluded that CMC universally had postdischarge problems that were able to be identified and managed through nurse home visits.29 In a large observational study, CMC discharged with home health nursing had fewer subsequent hospitalizations and 30-day readmissions compared with matched controls.30 Similarly, authors of a meta-analysis of clinical trials used to reduce readmissions found that postdischarge home visits and phone calls were most effective.31
Action planning has less supporting evidence for reducing hospitalizations outside of asthma. At 1 center, individualized pain plans for sickle cell crises were associated with fewer hospitalizations compared with 4 matched hospitals.32 Seizure action plans were developed in another center.33 Although it was not found that fewer hospitalizations occurred after seizure plan introduction, the plans were more narrowly focused on administering emergency antiepileptic drugs for prolonged seizures. Authors of a recent Cochrane review of “personalized care planning” for adults with chronic or long-term health conditions concluded that action planning improves health, self-management, and activation, particularly when the intervention is more comprehensive, intensive, and integrated into routine care.34
Although our study was not ultimately designed to determine the causal pathway of PACT, as an exploratory step, we are conducting a post hoc analysis to evaluate whether degree or nature of complexity moderates intervention effects on hospital use. Previous research reveals that having more CCCs is associated with higher hospital use,21 and certain CCCs are associated with persistent high spending year after year.35 If we observe that the effects of PACT are concentrated within certain subsets of children, 1 possible explanation is that action planning may be used to prevent the types of hospitalizations more commonly faced by those children. For example, children with devices might have hospitalizations that are more amenable to action planning than others if a large proportion of their hospitalizations are due to preventable device complications.
Lower mortality was observed with PACT post hoc. Although the intervention activities can be used to provide a plausible mechanism for at least 2 of the deaths, the intervention was not designed to reduce mortality. This finding requires confirmation in subsequent research before drawing conclusions. Because CMC–hospital use and spending commonly increases in the last year of life,36,37 it is notable that the 4 patients who died were neither the most expensive nor the most commonly hospitalized in the study.
PACT was selected because of its anticipated feasibility.14 On the basis of high intervention uptake, low dropout, and supportive qualitative comments, our data suggest caregivers felt the intervention was acceptable. The primary costs were salary to support the transition coach, home visit travel expenses (∼$79 680 during the same period), and costs for training in Colorado (∼$8000 for 2 coaches trained). Action planning was completed during regularly scheduled visits with existing clinical staff and office supplies, and therefore had only nominal costs. Action plans were discussed at weekly meetings, although this additional cost was not quantified. Programs with existing staff to implement PACT activities and those with newer payment models such as shared savings arrangements, may find this intervention more sustainable. For our program, total charges were reduced by nearly $1 million during the 18-month study.
Generalizability is an important limitation because PACT was implemented in a clinical program caring for predominantly publicly insured, urban lower income families from Hispanic, Spanish-speaking backgrounds. The feasibility of delivering the transition coaching intervention to a more rural population is not known but presumably more difficult if the coach and patients are located several hours from one another. Having bilingual and/or bicultural clinical and intervention staff was critical to the program’s success. An important next step will be to determine whether such findings are replicated in different populations and complex care clinical settings. Many programs are not medical homes, and it is unclear whether the effects would be similar in those focused on inpatient care or care coordination without primary care.
Although contamination of intervention activities to the usual care group threatened to diminish intervention effectiveness, we kept research staff offices and workflows distinct from clinical staff. We suspect that if usual care by providers was influenced indirectly by having other patients in PACT, it would have biased our results toward the null. Lastly, we only had access to data regarding hospital use from our institution and that which was reported to us from caregivers. Therefore, hospital use outside our institution was missed. Similarly, we were not able to conduct robust economic analyses because of the lack of complete intervention or health services cost data. With our data, we suggest that nearly 90% of all hospitalizations were identified by administrative sources and therefore provide a fairly complete picture.
Despite these limitations, our findings have several important implications. First, we demonstrate that even after enrollment in an established medical home with comprehensive care coordination, opportunities may exist to further reduce hospital use. Second, this is the first randomized controlled trial among CMC already receiving care within a complex care program, revealing the ability to conduct methodologically rigorous intervention research within this context. Limited power for many outcomes as well as the heterogeneity of clinical programs and CMC populations, however, underscore the need for collaborative, multisite research. Identifying the complex care key ingredients for improving outcomes most important to patients, families, health care providers, and payers is a critical step toward building a precise and efficient health system for our most vulnerable patients.
Glossary
- CCC
complex chronic conditions
- CI
confidence interval
- CMC
children with medical complexity
- CTI
care transitions intervention
- ED
emergency department
- IQR
interquartile range
- PACT
Plans for Action and Care Transitions
- RCT
randomized controlled trial
- UCLA
University of California, Los Angeles
Footnotes
Dr Coller conceptualized and designed the study, conducted primary data analysis, and drafted the initial manuscript; Dr Klitzner assisted with project conceptualization and data analysis and reviewed and revised the manuscript; Dr Lerner assisted with project conceptualization and reviewed and revised the manuscript; Dr Nelson assisted with data collection and analysis and reviewed and revised the manuscript; Ms Thompson and Ms Saenz coordinated and supervised data collection and critically reviewed the manuscript; Ms Zhao assisted in data management and assembly; Ms Ia assisted with project conceptualization and data interpretation and revised the manuscript; Ms Flores-Vazquez assisted with data interpretation and revised the manuscript; Dr Chung contributed to conceptualization, methodological supervision, data analysis, technical oversight, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02277327).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grant R40MC25677 Maternal and Child Health Research Program, Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services; by the National Institutes of Health National Center for Advancing Translational Science University of California, Los Angeles Clinical and Translational Science Institute grant UL1TR001881; and by funding to the Pediatric Medical Home Program at the University of California, Los Angeles from the Skirball Foundation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Berry JG, Hall M, Neff J, et al. . Children with medical complexity and Medicaid: spending and cost savings. Health Aff (Millwood). 2014;33(12):2199–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neff JM, Sharp VL, Muldoon J, Graham J, Myers K. Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coller RJ, Nelson BB, Sklansky DJ, et al. . Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6). Available at: www.pediatrics.org/cgi/content/full/134/6/e1628 [DOI] [PubMed] [Google Scholar]
- 4.Ralston SL, Harrison W, Wasserman J, Goodman DC. Hospital variation in health care utilization by children with medical complexity. Pediatrics. 2015;136(5):860–867 [DOI] [PubMed] [Google Scholar]
- 5.Mosquera RA, Avritscher EB, Samuels CL, et al. . Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648 [DOI] [PubMed] [Google Scholar]
- 6.Criscione T, Walsh KK, Kastner TA. An evaluation of care coordination in controlling inpatient hospital utilization of people with developmental disabilities. Ment Retard. 1995;33(6):364–373 [PubMed] [Google Scholar]
- 7.Gordon JB, Colby HH, Bartelt T, Jablonski D, Krauthoefer ML, Havens P. A tertiary care-primary care partnership model for medically complex and fragile children and youth with special health care needs. Arch Pediatr Adolesc Med. 2007;161(10):937–944 [DOI] [PubMed] [Google Scholar]
- 8.Klitzner TS, Rabbitt LA, Chang RK. Benefits of care coordination for children with complex disease: a pilot medical home project in a resident teaching clinic. J Pediatr. 2010;156(6):1006–1010 [DOI] [PubMed] [Google Scholar]
- 9.Liptak GS, Burns CM, Davidson PW, McAnarney ER. Effects of providing comprehensive ambulatory services to children with chronic conditions. Arch Pediatr Adolesc Med. 1998;152(10):1003–1008 [DOI] [PubMed] [Google Scholar]
- 10.Palfrey JS, Sofis LA, Davidson EJ, Liu J, Freeman L, Ganz ML; Pediatric Alliance for Coordinated Care . The Pediatric Alliance for Coordinated Care: evaluation of a medical home model. Pediatrics. 2004;113(suppl 5):1507–1516 [PubMed] [Google Scholar]
- 11.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207–214 [DOI] [PubMed] [Google Scholar]
- 12.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The medical home and hospital readmissions. Pediatrics. 2015;136(6). Available at: www.pediatrics.org/cgi/content/full/136/6/e1550 [DOI] [PubMed] [Google Scholar]
- 14.Coller RJ, Nelson BB, Klitzner TS, et al. . Strategies to reduce hospitalizations of children with medical complexity through complex care: expert perspectives. Acad Pediatr. 2017;17(4):381–388 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton LJ, Lerner CF, Presson AP, Klitzner TS. Effects of a medical home program for children with special health care needs on parental perceptions of care in an ethnically diverse patient population. Matern Child Health J. 2013;17(3):463–469 [DOI] [PubMed] [Google Scholar]
- 16.Nelson BB, Coller RJ, Saenz AA, et al. . How avoidable are hospitalizations for children with medical complexity? Understanding parent perspectives. Acad Pediatr. 2016;16(6):579–586 [DOI] [PubMed] [Google Scholar]
- 17.Zemek RL, Bhogal SK, Ducharme FM. Systematic review of randomized controlled trials examining written action plans in children: what is the plan? Arch Pediatr Adolesc Med. 2008;162(2):157–163 [DOI] [PubMed] [Google Scholar]
- 18.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828 [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494 [DOI] [PubMed] [Google Scholar]
- 21.Berry JG, Hall DE, Kuo DZ, et al. . Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feudtner C, Levin JE, Srivastava R, et al. . How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry JG, Agrawal RK, Cohen E, Kuo DZ. The Landscape of Medical Care for Children With Medical Complexity. Alexandria, VA: Children’s Hospital Association; 2013 [Google Scholar]
- 25.Cohen E, Kuo DZ, Agrawal R, et al. . Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry C, Min SJ, Chugh A, Chalmers S, Coleman EA. Further application of the care transitions intervention: results of a randomized controlled trial conducted in a fee-for-service setting. Home Health Care Serv Q. 2009;28(2–3):84–99 [DOI] [PubMed] [Google Scholar]
- 27.Parrish MM, O’Malley K, Adams RI, Adams SR, Coleman EA. Implementation of the care transitions intervention: sustainability and lessons learned. Prof Case Manag. 2009;14(6):282–293; quiz 294–295 [DOI] [PubMed] [Google Scholar]
- 28.Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2–11 [DOI] [PubMed] [Google Scholar]
- 29.Wells S, O’Neill M, Rogers J, et al. . Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10–16 [DOI] [PubMed] [Google Scholar]
- 30.Gay JC, Thurm CW, Hall M, et al. . Home health nursing care and hospital use for medically complex children. Pediatrics. 2016;138(5):e20160530. [DOI] [PubMed] [Google Scholar]
- 31.Branowicki PM, Vessey JA, Graham DA, et al. . Meta-analysis of clinical trials that evaluate the effectiveness of hospital-initiated postdischarge interventions on hospital readmission. J Healthc Qual. 2017;39(6):354–366 [DOI] [PubMed] [Google Scholar]
- 32.Krishnamurti L, Smith-Packard B, Gupta A, Campbell M, Gunawardena S, Saladino R. Impact of individualized pain plan on the emergency management of children with sickle cell disease. Pediatr Blood Cancer. 2014;61(10):1747–1753 [DOI] [PubMed] [Google Scholar]
- 33.Roundy LM, Filloux FM, Kerr L, Rimer A, Bonkowsky JL. Seizure action plans do not reduce health care utilization in pediatric epilepsy patients. J Child Neurol. 2016;31(4):433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;(3):CD010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal R, Hall M, Cohen E, et al. . Trends in health care spending for children in Medicaid with high resource use. Pediatrics. 2016;138(4):e20160682. [DOI] [PubMed] [Google Scholar]
- 36.Ananth P, Melvin P, Feudtner C, Wolfe J, Berry JG. Hospital use in the last year of life for children with life-threatening complex chronic conditions. Pediatrics. 2015;136(5):938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindley LC, Lyon ME. A profile of children with complex chronic conditions at end of life among Medicaid beneficiaries: implications for health care reform. J Palliat Med. 2013;16(11):1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]