Abstract
Tolerance and associated hyperalgesia induced by long-term morphine administration substantially restrict the clinical use of morphine in pain treatment. Melatonin, a neurohormone released by the pineal gland, has been demonstrated to attenuate anti-nociceptive morphine tolerance. The present study investigates differentially expressed genes in the process of morphine tolerance and altered gene expression subsequent to melatonin treatment in chronic morphine-infused ratspinal cords. Morphine tolerance was induced in male Wistar rats by intrathecal morphine infusion (the MO group). Melatonin (the MOMa group) was administered to overcome the effects derived by morphine. The mRNA collected from L5-S3 of the spinal cord was extracted and analysed by rat expression microarray. Principal component analysis and clustering analysis revealed that the overall gene profiles were different in morphine and melatonin treatments. Subsequent to Gene Ontology analysis, the biological processes of differentially expressed genes of MO and MOMa compared with the control group were constructed. Furthermore, a panel of genes exclusively expressed following melatonin treatment and another panel of genes with inverse expression between the MO and MOMa group were also established. Subsequent to PANTHER pathway analysis, a group of genes with inverse expression following melatonin administrated compared with morphine alone were identified. The expression levels of genes of interest were also confirmed using a reverse transcription-quantitative polymerase chain reaction. The gene panel that was constructed suggests a potential signaling pathway in morphine tolerance development and is valuable for investigating the mechanism of morphine tolerance and the regulatory gene profiles of melatonin treatment. These results may contribute to the discovery of potential drug targets in morphine tolerance treatments in the future.
Keywords: melatonin, morphine tolerance, gene expression
Introduction
Morphine is a powerful analgesic agent used for treating acute and chronic pain in surgical interventions or in hospice care (1). However, long-term administration of morphine induces tolerance and hyperalgesia. Furthermore, adverse effects, including addiction, dependence, constipation and respiratory depression limit its clinical usefulness (2,3). The physiological responses of morphine tolerance include opioid receptor uncoupling, endocytosis/desensitization (4), increased binding of β-arrestin to opioid receptors, glutamatergic receptor activation and neuroinflammation (5). Melatonin is a neurohormone derived from serotonin and is released from the pineal gland (6). It is used for sleep modulation and relieves the stress caused by sleep disturbance (1). It has previously been revealed that melatonin treatment partially reverses morphine tolerance by inhibiting microglia activation though a heat shock protein 27 (HSP27)-associated pathway (7). Furthermore, melatonin co-treatment was revealed to prevent morphine-induced hyperalgesia and tolerance in rats, potentially by inhibiting protein kinase C-associated pathways (8,9). A report also demonstrated that decreased mitochondrial DNA copy numbers in the hippocampus during opiate addiction were mediated by autophagy and may be reversed by melatonin (10). Additionally, melatonin was revealed to enhance the reward behaviour of morphine via the nitric oxidergic pathway (11). Raghavendra and Kulkarni initially reported that the systemic administration of melatonin reversed morphine-induced tolerance in mice (12). Song et al (8) identified that daily intraperitoneal melatonin treatment reduced morphine tolerance in rats via the regulation of the N-methyl-D-aspartate receptor subunit 1. Furthermore, Garmabi et al (13) observed a reduction of melatonin levels in rats under constant light exposure; those animals also presented a high morphine consumption and severe morphine withdrawal syndrome. Fan et al (14) further reported a substantial decrease of serum melatonin and melatonin receptor 1 mRNA subsequent to chronic morphine infusion in rats. Previously, not only was it revealed that melatonin treatment partially reversed morphine tolerance by inhibiting microglia activation though a HSP27-associated pathway (7), but preliminary examinations additionally revealed that chronic morphine treatment resulted in transcriptomics changes. All studies noted that melatonin participates in the morphine tolerance pathway. Although melatonin was demonstrated to diminish morphine tolerance, the transcriptomic changes derived from melatonin treatment in opiate tolerance remain undetermined. To search whole genome expression profiles disturbed by long-term morphine administration and clarify the gene alterations caused by melatonin, an expression array was used in the present study to examine the effects of melatonin treatment on morphine-induced tolerance in rats. The results may provide insight on and contribute to deciphering the detailed mechanisms of morphine tolerance.
Materials and methods
Construction of intrathecal catheters
The intrathecal (i.t.) catheters were constructed by inserting a 3.5 cm Silastic tube (Corning Incorporated, Corning, NY, USA) into an 8 cm polyethylene tube (0.008 inch internal diameter, 0.014 inch outer diameter; Spectranetics, Colorado Springs, CO, USA) and sealing the joint with epoxy resin and silicon rubber as previously described (15).
Animal preparation and intrathecal drug delivery
The use of rats in the present study adhered to the Guiding Principles in the Care and Use of Animals of the American Physiology Society (16) and was ethically approved by the National Defense Medical Center Animal Care and Use Committee (Taipei, Taiwan). A total of 27 Male Wistar rats (350-400 g), each rat (with 12 weeks of age) was housed individually at a room temperature at 25°C, at 1 atm, with water and food freely as wish. The rats were anaesthetized with phenobarbital (65 mg/kg, intraperitoneally) and two i.t. catheters were implanted. The catheters were inserted via the atlantooccipital membrane down to spinal cord segments L5, L6 and S1-S3, which are associated with the tail-flick reflex (17). One catheter was connected to a mini-osmotic pump (Alzet, Cupertino, CA, USA) for an infusion of saline or morphine (15 µg/h) for 7 days at a rate of 1 µl/h. Subsequent to cath-eterization (day 0), the rats were returned to their home cages and maintained in a 12 h light/dark cycle with ad libitum access to food and water. Rats with neurological deficits were excluded. On day 7, by which time a morphine tolerance had developed, the catheter used for saline or morphine infusion was cut and blocked with a metal metal plug to prevent CSF leakage. The rats were injected i.t. via the second catheter with 5 µl either with vehicle (10% ethanol) or melatonin (50 µg in 10% ethanol), then, 30 min later, a single dose of morphine (15 µg in 5 µl saline, i.t.) was injected and the antinociceptive effect measured. The protocol is presented in Fig. 1A. There were four experimental groups used in the present study, as follows: Controls, melatonin-treated, morphine-treated and those treated with melatonin and morphine combined. For the control group, the animals were infused with saline for 7 days and infused with vehicle injection for 30 min, and subsequently injected with saline. For the morphine group, the animals were infused with morphine for 7 days, injected with vehicle injection for 30 min and subsequently injected with morphine. For the melatonin group, the animals were infused with morphine for 7 days and injected with melatonin for 30 min, and subsequently injected with saline. For the melatonin and morphine group, the animals were infused with morphine for 7 days, injected with melatonin for 30 min and subsequently injected with morphine.
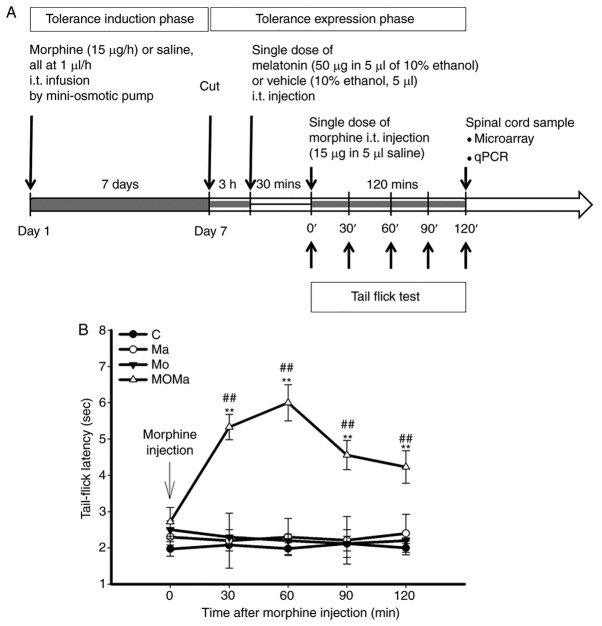
Figure 1.
Experimental procedure and effect of melatonin on the antinocieptive effect in morphine-tolerant rats. (A) Experimental procedure for drug administration. Male Wistar rats were implanted with two i.t. catheters, one of which was connected to a mini-osmotic pump for the infusion of morphine or saline for 7 days. On day 7, subsequent to morphine tolerance development, the catheter was cut, and 3 h later the rats were injected intrathecally with either vehicle or melatonin via the second catheter. A total of 30 min later, a single dose of morphine (15 µg) was injected intrathecally and its antinociceptive effect measured. (B) Melatonin reverses the antinociceptive effect of morphine in morphine-tolerant rats. Antinociception of morphine was assessed on day 7 following intrathecal infusion of saline or morphine. At 3 h subsequent to the discontinuation of infusion, the rats were injected intrathecally with 10% ethanol (as vehicle) or 50 µg melatonin. After 30 min, the rats underwent a 15 µg morphine administration, then tail-flick latency was measured every 30 min for 120 min. All data are presented as the mean ± standard error of the mean for at least 5 rats. **P<0.01 vs. the Ma group; ##P<0.01 vs. the MO group. C, control (saline infusion/vehicle injection/saline challenge); MO, morphine (morphine infusion/vehicle injection/morphine challenge); Ma, melatonin (morphine infusion/melatonin injection/saline challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge); i.t., intrathecal; qPCR, quantitative polymerase chain reaction.
The dose of morphine selected was based on a previous study (18). For i.t. injection, melatonin was dissolved in ethanol (50 µg/5 µl in 10% ethanol maximum). All drugs were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and were delivered i.t., followed by the flushing of the catheter with 5 µl saline. Preliminary results revealed no abnormal motor function subsequent to i.t. injection of the test drugs (data not shown).
Antinociception test
Tail-flick latency was measured using the hot water immersion test (52±0.5°C). Baseline latency was ~2±0.38 sec, and a cutoff time of 10 sec was used. Rats were placed in plastic restrainers for drug injection and antinociception testing.
Spinal cord sample collection from rats with different treatments
Spinal cord sample collection was performed as previously described (7), and morphine tolerance in rats was confirmed by the time-course of tail-flick latency over a 7-day period. Prior to day 4, the rats with morphine infusions demonstrated a reduction of tail-flick latency compared with the saline-infused group, which exhibited no changes in latency during the period. Substantial morphine tolerance was developed on day 7 as determined by a significant reduction of the antinociceptive effect of morphine compared with day one, with a reduction of the tail-flick latency of ~60%. And then, rats were i.t. injected with either 10% ethanol (as a vehicle) or melatonin via the externalized i.t. catheter. A total of 30 min later, a single dose of morphine (15 µg) was injected i.t. to confirm morphine tolerance. In contrast, melatonin pretreatment attenuated morphine tolerance, melatonin pretreatment was done by administering melatonin on day 7, at 30 min prior to morphine intrathecal injection. The lumbar enlargement segment was removed from 4 rat spinal cords from each group for differential gene expression analysis.
Spinal cord sample preparation
Following drug treatment, the rats were sacrificed by exsanguination under anaesthesia with isoflurane (Abbott Pharmaceutical Co. Ltd., Lake Bluff, IL, USA) and a laminectomy was performed at the lower edge of the 12th thoracic vertebra. Subsequently, the lumber enlargement (L5-S3) of the spinal cord was immediately collected for subsequent analysis.
Rat expression microarray
Following the tail-flick test, the rats were sacrificed, and lumber enlargement (L5-S3) of the spinal cord was immediately collected. There were 4 samples tested in each group. Total mRNAs were extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA concentration and purity were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA) with a criteria of OD260/OD280 (>1.8) and OD260/OD230 (>1.6). Next, the RNAs were labelled with Cy5 dye by an indirect NHS ester labelling kit (GE Healthcare, Chicago, IL, USA) according to the manufacturer’s protocol. The labelled RNAs were hybridized with a Rat OneArray® microarray (Phalanx Biotech Group, Hsinchu, Taiwan), which contains 24,358 rat genome probes and 980 experimental control probes. All the probes correspond to annotated genes in RefSeq and Ensembl databases. The hybridization procedure was performed at 50°C in a Phalanx Hybridization System (Phalanx Biotech Group). A total of 16 h after hybridization, non-specific binding targets were washed away using three sequential washing steps by 2X saline-sodium citrate buffer (SSC) contained 0.2% SDS solution for 5 min at 42°C. Then, the slide was spun dry with a centrifuge for 1 min at room temperature. The images of the microarray were scanned using an Agilent G2505C scanner (Agilent Technologies, Inc.). The Cy5 fluorescence intensities of each spot were analysed by GenePix 4.1 software (Molecular Devices, LLC, Sunnyvale, CA, USA).
Microarray analysis
Microarray spot analysis was resolved by the Rosetta Resolver System® (Rosetta Biosoftware, Seattle, WA, USA). Control probes data were calculated, and the reproducibility of each microarray slide was assessed using Pearson’s correlation coefficient calculations with a criterion of R-value >0.975. Normalized spot intensities were transformed to gene expression log2 ratios in each group. For further analysis, the spots with a log2 ratio ≥1 or a log2 ratio ≤−1 or undetectable log2 ratios but with differences in intensity between the two samples of >1,000 and a P<0.05 were selected according to the method of Pirooznia et al (19). Principal Component Analysis (PCA) was performed to evaluate any differences among biological replicates and their treatment conditions using FDA released ArrayTrack™ HCA-PCA Standalone Package (20). PCA uses an orthogonal transformation to convert a set of observations of possibly correlated variables into a set of values of uncorrelated variables called principal components. For advanced data analysis, intensity data were pooled and calculated to identify differentially expressed genes based on the threshold of fold-change and P-value. The correlation of expression profiles between samples and treatment conditions was demonstrated by unsupervised hierarchical clustering analysis. Average linkage clustering was performed to visualize the correlations among the replicates and varying sample conditions using and open source software, Java Treeview (21). Up and downregulated genes are represented in red and green colors, respectively.
Gene ontology (GO) enrichment analysis
The gene IDs of interest were uploaded to the Gene ontology Enrichment analysis website (22). The database and analysis services were funded by the National Human Genome Research Institute in the U.S. And right now the website were maintained and updated by the Gene Ontology Consortium (GOC). The names of the genes with interested were paste to the query column in the website and set the GO aspect as molecular function for the analysis. The database search was confined to Rattus norvegicus database.
Gene pathway mapping by PANTHER
The gene IDs of interest were uploaded to the PANTHER Classification System website (http://pantherdb.org). PANTHER is a comprehensive, curated database of protein families, trees, subfamilies, functions and ontology (23). The search parameter was set to ‘molecular function’, and the database search was confined to only the Rattus norvegicus database. The keywords used were the gene names of interest and the access date were December 12 and 19, 2017.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Tissues were collected as described above. RNA was extracted within 1 h at room temperature using TRIzol reagent following manufacturer’s protocol (Invitrogen; Thermo Fisher Scientific, Inc.). mRNA were reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA were amplified and subjected to optical analysis to verify the integrity of extracted RNA. The expression of target genes were quantified for all experimental groups using LightCycler system (Roche Diagnostics, Basel, Switzerland). RT-qPCR analysis thermocycling conditions were: 95°C for 10 min and then the cycling conditions were set as 95°C for 10 sec, 60°C for 20 sec, 72°C for 40 sec for 50 cycles. The method of quantification for RT-qPCR products were followed Livak and Schmittgen et al (24) The relative abundance of transcripts were normalized to the constitutive expression of GAPDH. The primers of each genes used in RT-qPCR were listed in Table I.
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction analysis.
| Target gene | Gene ID | Position | Sequence |
|---|---|---|---|
| G protein subunit β 1 (Gnb1) | NM_030987.2 | F:262-281 | 5′-tccagtgggaagaatccaaa-3′ |
| R:317-337 | 5′-ccagtgcatggcataaatctt-3′ | ||
| Cholecystokinin B receptor (Cckbr) | NM_013165.2 | F:1799-1819 | 5′-cccgtttgacttcattattgc-3′ |
| R:1842-1861 | 5′-tgaaaggcgtgtggttgata-3′ | ||
| 5-hydroxytryptamine receptor 1A (Htr1a) | NM_012585.1 | F:1054-1072 | 5′-ggcaccttcatcctctgct-3′ |
| R:1110-1128 | 5′-gtggcagctgctttcacag-3′ | ||
| RAS protein activator like 1 (Rasal1) | NM_001108335.1 | F:408-429 | 5′-ggagtacactgttcaccttcca-3′′ |
| R:451-470 | 5′-tcctcatccagcacgtagaa-3′ | ||
| General transcription factor 2A subunit 1 like (Gtf2a1l) | NM_001012136.1 | F:1222-1242 | 5′-gaggatcccctaaattctgga-3′ |
| R:1267-1289 | 5′-ttatctgtgtcaaacaggtctgg-3′ | ||
| Period circadian clock 1 (Per1) | NM_001034125.1 | F:1986-2008 | 5′-tcctaacacaaccaagcgtaaat-3′ |
| R:2043-2062 | 5′-ccctctgcttgtcatcatca-3′ | ||
| Methionine adenosyltransferase 2A (Mat2a) | NM_134351.1 | F:149-168 | 5′-tgtaggggaaggtcatccag-3′ |
| R:204-222 | 5′-cctgctgaaggtgtgcatc-3′ | ||
| Collagen type V α 3 chain (Col5a3) | NM_021760.1 | F:634-652 | 5′-cggggaggagtcttttgag-3′ |
| R:673-693 | 5′-gcctgagggtctggaattaac-3′ | ||
| Inositol 1,4,5-trisphosphate receptor, type 3 (Itpr3) | NM_013138.1 | F:8362-8381 | 5′-taggggatgcaagttctcca-3′ |
| R:8403-8422 | 5′-ccactgagaaatgccagtca-3′ | ||
| Diacylglycerol kinase ζ (Dgkz) | NM_031143.1 | F:330-347 | 5′-ctttgggcacaggaaagc-3′ |
| R:410-429 | 5′-gatctgccgctcagattcac-3′ | ||
| LIM zinc finger domain containing 2 (Lims2) | NM_001012163.1 | F:966-985 | 5′-tcatgtgattgagggtgacg-3′ |
| R:1032-1051 | 5′ccaccaggagaacagactgg-3′ |
F, forward; R, reverse.
Data and statistical analyses
All data are presented as the mean ± standard error of the mean. Statistical analysis was performed using SigmaStat 3.0 software (SYSTAT Software Inc., San Jose, CA, USA). Tail-flick latencies were analyzed using two-way (time and treatment) analysis of variance (ANOVA), followed by one-way ANOVA with a post hoc Student-Newman-Keuls test. The RT-qPCR results were analyzed using a Student’s t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Experimental design and procedure
The experimental procedure for drug administration was depicted in Fig. 1. Male Wistar rats were implanted with two i.t. catheters and connected to a mini-osmotic pump for morphine or saline infusion for 7 days for morphine tolerance induction. On day 7, subsequent to the development of tolerance, the catheter was cut, and 3 h later, the rats received an i.t. injection of either vehicle or melatonin via the other catheter. A total of 30 min later, at the tolerance expression phase, a single dose of morphine (15 µg) was injected i.t., and the antinociceptive effect was measured. Tail flick tests were performed in every experimental group and the results were presented in Fig. 1B. There was a significant reduction of morphine tolerance subsequent to melatonin addition compared with the control group (P<0.01). Following a tail-flick test, the rats were sacrificed, and the L5 to S3 region of the spinal cords were collected for further analysis.
Differential gene expression among morphine tolerance, melatonin treatment and morphine tolerance combined with melatonin treatment groups
To determine the alterations in gene expression caused by morphine and reversed by melatonin treatment in rat spinal cords, rat global gene expression profiles of four independent RNA samples from each group were selected for microarray analysis. PCA and clustering analysis revealed that the overall gene profiles derived from microarray analysis were separated based on morphine or melatonin treatment. The result revealed that 162 genes were upregulated and 16 genes were downregulated in the morphine-tolerant group (MO group, n=7) compared with the control group (C group, n=5); 476 genes were upregulated and 71 genes were downregulated in the melatonin treatment group (Ma group, n=5) compared with the control group (C group), and 290 genes were upregulated and 15 genes were downregulated in the morphine with acute melatonin treatment group (MOMa group, n=10) compared with the control group (Table II). All genes selected using the criteria of log2|Fold change|≥ 1 and P<0.05 or undetectable log2 ratios but with differences in intensity between the two samples of >1,000. Statistical significance was used to avoid confounding due to variation amongst the animals and addressed additional evidence that the transcriptional profiles of morphine tolerance and melatonin treatment in vivo are different. The present study compared the number of upregulated genes between the MO and MOMa groups; it was identified that the number of upregulated genes in the MOMa group was greater compared with the number in the MO group, which indicated that melatonin restored the antinociceptive effect of morphine, which was accomplished with multiple gene expression alterations.
Table II.
Number of differentially expressed genes.
| Group comparison | Upregulated | Downregulated |
|---|---|---|
| MO/C | 162 | 16 |
| MOMa/C | 290 | 15 |
| Ma/C | 476 | 71 |
Standard selection criteria to identify differentially expressed genes are as follows: i) log2 |Fold change|≥1 and P<0.05; ii) log2 ratios=‘NA’ and the differences of intensity between the two samples ≥1000. Detail gene lists were be provided by request. C, control (saline infusion/vehicle injection/saline challenge); Mo, morphine (morphine infusion/vehicle injection/morphine challenge); Ma, melatonin (morphine infusion/melatonin injection/saline challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge).
Gene ontology (GO) analysis of the altered genes from the MO or MOMa group
The differentially expressed genes were then subjected to GO analysis based on molecular function (Table III). Numerous GO terms were identical between the two groups; however, opsonin binding, actin binding, calcium ion binding, sugar binding, oxidase activity, deaminase activity, protein complex binding and oxidoreductase activity were not identified in the MO group. On the other hand, GTPase activity, phospholipase inhibitor activity, cytokine activity, GTP binding, guanyl-nucleotide and ribonucleotide binding, immunoglobulin (Ig)G receptor activity, IgE binding and protein dimerization activity were not identified in the MOMa group, implying the potential regulatory mechanism of melatonin treatment. From the GO terms identified between the MO and MOMa groups, it was revealed that a number of notable pathways were altered. It has been previously reported that the morphine tolerance process involves inflammation (25). Immune-associated processes, including cytokine activity, IgG receptor activity and IgE binding, were missing following melatonin treatment, which indicates that melatonin treatment may participate in the downregulation of these cellular process. On the other hand, the gene expression for actin binding was present following melatonin treatment; this result implies that cytoskeleton reconstitution may be activated. Additionally, genes involving calcium ion binding, sugar binding, NADPH oxidase activity, deaminase activity and protein complex binding pathways appeared subsequent to melatonin treatment, indicating the requirement for the metabolic activity that emerged following melatonin treatment. The gene expression data for IgG receptor expression and actin binding were selected and provided by request.
Table III.
Identified gene ontology terms of the MO and MOMa groups compared with the C group.
| Geneset name | MO/C
|
MOMa/C
|
||||
|---|---|---|---|---|---|---|
| No. of genes in geneset | No. of genes in overlap | P-value | No. of genes in geneset | No. of genes in overlap | P-value | |
| GO:0001846-opsonin binding | N.I. | 7 | 3 | <0.01 | ||
| GO:0001871-pattern binding | 116 | 7 | <0.01 | 116 | 13 | <0.01 |
| GO:0001872-zymosan binding | 3 | 2 | 0.02 | 3 | 2 | 0.04 |
| GO:0003924-GTPase activity | 98 | 4 | 0.05 | N.I. | ||
| GO:0003779-actin binding | N.I. | 233 | 10 | 0.01 | ||
| GO:0004857-enzyme inhibitor activity | 238 | 10 | <0.01 | 238 | 11 | <0.01 |
| GO:0004859-phospholipase inhibitor activity | 6 | 2 | 0.05 | N.I. | ||
| GO:0004866-endopeptidase inhibitor activity | 148 | 6 | 0.01 | 148 | 7 | 0.02 |
| GO:0005125-cytokine activity | 110 | 5 | 0.01 | N.I. | ||
| GO:0005506-iron ion binding | 289 | 7 | 0.03 | 289 | 10 | 0.03 |
| GO:0005525-GTP binding | 312 | 8 | 0.02 | N.I. | ||
| GO:0005509-calcium ion binding | N.I. | 672 | 20 | 0.01 | ||
| GO:0005529-sugar binding | N.I. | 215 | 9 | 0.02 | ||
| GO:0005539-glycosaminoglycan binding | 102 | 5 | 0.01 | 102 | 11 | <0.01 |
| GO:0008009-chemokine activity | 32 | 5 | <0.01 | 32 | 4 | 0.01 |
| GO:0008201-heparin binding | 72 | 4 | 0.02 | 72 | 8 | <0.01 |
| GO:0016175-superoxide-generating | N.I. | 7 | 3 | <0.01 | ||
| NADPH oxidase activity | ||||||
| GO:0016814~hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds, in cyclic amidines | 22 | 3 | 0.01 | 22 | 4 | <0.01 |
| GO:0019239-deaminase activity | N.I. | 21 | 3 | 0.04 | ||
| GO:0019001-guanyl nucleotide binding | 326 | 8 | 0.02 | N.I. | ||
| GO:0019763-immunoglobulin receptor activity | 7 | 3 | <0.01 | 7 | 3 | <0.01 |
| GO:0019770-IgG receptor activity | 4 | 2 | 0.03 | N.I. | ||
| GO:0019834-phospholipase A2 inhibitor activity | 3 | 2 | 0.02 | 3 | 2 | 0.04 |
| GO:0019863-IgE binding | 4 | 2 | 0.03 | N.I. | ||
| GO:0019864-IgG binding | 6 | 4 | <0.01 | 6 | 4 | <0.01 |
| GO:0019865-immunoglobulin binding | 12 | 5 | <0.01 | 12 | 5 | <0.01 |
| GO:0019955-cytokine binding | 87 | 4 | 0.04 | 87 | 7 | <0.01 |
| GO:0020037-heme binding | 148 | 5 | 0.04 | 148 | 7 | 0.02 |
| GO:0030246-carbohydrate binding | 337 | 10 | <0.01 | 337 | 20 | <0.01 |
| GO:0030247-polysaccharide binding | 116 | 7 | <0.01 | 116 | 13 | <0.01 |
| GO:0030414-peptidase inhibitor activity | 159 | 7 | <0.01 | 159 | 8 | 0.01 |
| GO:0032403-protein complex binding | N.I. | 222 | 12 | <0.01 | ||
| GO:0032561-guanyl ribonucleotide binding | 326 | 8 | 0.02 | N.I. | ||
| GO:0042379-chemokine receptor binding | 33 | 5 | <0.01 | 33 | 4 | 0.01 |
| GO:0042802-identical protein binding | 588 | 11 | 0.02 | 588 | 18 | 0.01 |
| GO:0042803-protein homodimerization activity | 318 | 8 | 0.02 | 318 | 15 | <0.01 |
| GO:0046906-tetrapyrrole binding | 154 | 5 | 0.04 | 154 | 7 | 0.03 |
| GO:0046983-protein dimerization activity | 528 | 10 | 0.03 | 528 | 20 | <0.01 |
| GO:0048020-CCR chemokine receptor binding | 3 | 2 | 0.02 | N.I. | ||
| GO:0050664-oxidoreductase activity, acting on NADH or NADPH, with oxygen as acceptor | N.I. | 11 | 3 | 0.01 | ||
N.I., not identified; Ig, immunoglobulin; GO, Gene Ontology; C, control (saline infusion/vehicle injection/saline challenge); Mo, morphine (morphine infusion/vehicle injection/morphine challenge); Ma, melatonin (morphine infusion/melatonin injection/saline challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge).
Venn diagram and genes exclusively expressed in the MOMa group
In order to clarify the differential gene expression panels among the three groups, Venn diagram analysis was performed, and the results depicted the overlap of differentially expressed genes between the MO, Ma and MOMa groups (Fig. 2). In total, 48 genes were upregulated and 8 were genes downregulated exclusively in the MOMa group. These genes were the candidates that participated in the reversal of morphine tolerance. It was also identified that 20 genes were upregulated and 13 genes were downregulated exclusively in the MO group; these genes were not altered by melatonin treatment, so these genes were not involved in the melatonin reversal effect in morphine tolerance. All the genes in Venn diagram analysis are listed in Table IV-A and -B. Genes expressed exclusively in the MOMa group are notable as they may be the targets for the reversal of morphine tolerance associated with melatonin in future studies. From Table IV-B, the myocilin gene demonstrated the greatest fold change in upregulation; and myocilin has been reported to mediate myelination in the peripheral nervous system (26). Furthermore, microtubule-associated protein 9 expression was decreased in the MOMa group, and this gene has been reported to serve a role in mitotic spindle formation and mitosis progression (27), implying the potential involvement of melatonin.
Figure 2.
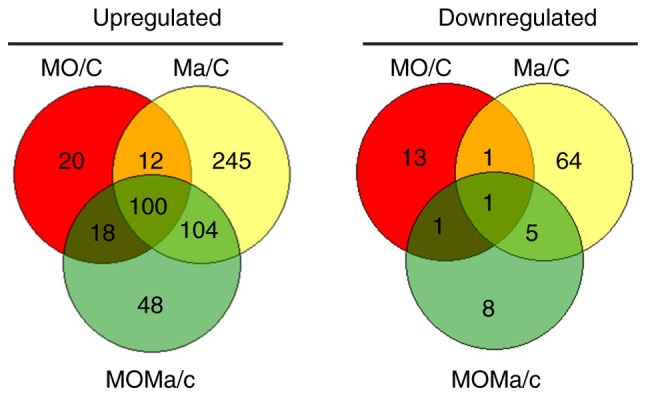
Venn diagram analysis of the genes that were upregulated (left) or downregulated (right) by morphine tolerance and/or melatonin treatment compared with the control group. In each pair test, the upregulated genes were identified as follows: Log2 |Fold change| ≥ 1 and P<0.05 In the diagram, red circles represent genes putatively affected by long-term morphine application, yellow circles represent genes influenced by melatonin treatment and green circles represent genes for which melatonin-induced expression changes were putatively affected by morphine. All genes considered were differentially expressed compared with the untreated group. C, control (saline infusion/vehicle injection/saline challenge); MO, morphine (morphine infusion/vehicle injection/morphine challenge); Ma, melatonin (morphine infusion/melatonin injection/saline challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge).
Table IV.
Top 20 exclusively upregulated and downregulated genes in each group.
| A, Exclusively expressed genes in MO group
| |||
|---|---|---|---|
| Gene symbol | Description | Gene ID | Fold-change |
| Ddx60 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 60, probable ATP-dependent RNA helicase DDX60-like | 100360801 | 1.61 Up |
| Lgals3bp | Lectin, galactoside-binding, soluble, 3 binding protein | 245955 | 1.54 Up |
| Oas1a | 2′-5′ oligoadenylate synthetase 1A | 192281 | 1.47 Up |
| Isg15 | ISG15 ubiquitin-like modifier | 298693 | 1.46 Up |
| Slamf9 | SLAM family member 9 | 289235 | 1.29 Up |
| Usp18 | Ubiquitin specific peptidase 18 | 312688 | 1.28 Up |
| Smim5 | Small integral membrane protein 5 | 689926 | 1.26 Up |
| Casp4 | Caspase 4, apoptosis-related cysteine peptidase | 114555 | 1.2 Up |
| Cd33 | CD33 molecule | 690492 | 1.2 Up |
| Pik3ap1 | Phosphoinositide-3-kinase adaptor protein 1 | 294048 | 1.14 Up |
| Ccl7 | Chemokine (C-C motif) ligand 7 | 287561 | 1.13 Up |
| Apol9a | Apolipoprotein L 9a | 503164 | 1.11 Up |
| Dpt | Dermatopontin | 289178 | 1.09 Up |
| Cryaa | Crystallin, αA | 24273 | 1.06 Up |
| Irgm | Immunity-related GTPase family, M | 303090 | 1.05 Up |
| Irf7 | Interferon regulatory factor 7 | 293624 | 1.04 Up |
| Gpr160 | G protein-coupled receptor 160 | 499588 | 1.03 Up |
| Uba7 | Ubiquitin-like modifier activating enzyme 7 | 301000 | 1.03 Up |
| Vwa5b1 | Von Willebrand factor A domain containing 5B1 | 313653 | 1.03 Up |
| Olr104 | Olfactory receptor 104 | 293243 | 1.02 Up |
| LOC689064 | β-globin | 689064 | −1 Down |
| Fras1 | Fraser syndrome 1 | 289486 | −1.01 Down |
| Zfp597 | Zinc finger protein 597 | 266774 | −1.06 Down |
| LOC681849 | Similar to protein C6orf142 homolog | 681849 | −1.09 Down |
| Alas2 | Aminolevulinate, delta-, synthase 2 | 25748 | −1.13 Down |
| LOC500300 | Similar to hypothetical protein MGC6835 | 500300 | −1.2 Down |
| Hspa1b | Heat shock 70 kD protein 1B (mapped) | 294254 | −1.21 Down |
| Ccdc77 | Coiled-coil domain containing 77 | 312677 | −1.3 Down |
| Oas1e | 2′-5′ oligoadenylate synthetase 1E | 494201 | −1.4 Down |
| Pmp2 | Peripheral myelin protein 2 | 688790 | −1.47 Down |
| Fkbp6 | FK506 binding protein 6 | 288597 | −1.98 Down |
| Prx | Periaxin | 78960 | −2.16 Down |
| Mpz | Myelin protein zero | 24564 | −2.92 Down |
| B, Exclusively expressed genes in MOMa group
| |||
|---|---|---|---|
| Gene symbol | Description | Gene ID | Fold-change |
| Myoc | Myocilin | 81523 | 2.47 Up |
| Samsn1 | SAM domain, SH3 domain and nuclear localization signals, 1 | 170637 | 1.48 Up |
| Scin | Scinderin | 298975 | 1.45 Up |
| Ncan | Neurocan | 58982 | 1.35 Up |
| Tagln | Transgelin | 25123 | 1.34 Up |
| Aplnr | Apelin receptor | 83518 | 1.31 Up |
| Nlrc4 | NLR family, CARD domain containing 4 | 298784 | 1.28 Up |
| S1pr3 | Sphingosine-1-phosphate receptor 3 | 306792 | 1.27 Up |
| Mxra8 | Matrix-remodelling associated 8 | 313770 | 1.26 Up |
| Sptbn5 | Spectrin, β, non-erythrocytic 5 | 296090 | 1.24 Up |
| Plin2 | Perilipin 2 | 298199 | 1.23 Up |
| Epyc | Epiphycan | 314772 | 1.23 Up |
| Chdh | Choline dehydrogenase | 290551 | 1.22 Up |
| Hlx | H2.0-like homeobox | 364069 | 1.19 Up |
| Cenpf | Centromere protein F | 257649 | 1.19 Up |
| Aoah | Acyloxyacyl hydrolase (neutrophil) | 498757 | 1.17 Up |
| Spta1 | Spectrin, α, erythrocytic 1 (elliptocytosis 2) | 289257 | 1.15 Up |
| Trim47 | Tripartite motif-containing 47 | 690374 | 1.14 Up |
| Abi3 | ABI family, member 3 | 303476 | 1.13 Up |
| Ssc5d | Scavenger receptor cysteine rich domain containing (5 domains) | 308341 | 1.13 Up |
| Epm2aip1 | EPM2A (laforin) interacting protein 1 | 316021 | −1.02 Down |
| LOC691921 | Hypothetical protein LOC691921 | 691921 | −1.04 Down |
| Klhl11 | Kelch-like 11 (Drosophila) | 287706 | −1.09 Down |
| Ppargc1b | Peroxisome proliferator-activated receptor γ, coactivator 1 β | 291567 | −1.11 Down |
| Pcdhb6 | Protocadherin β6 | 291653 | −1.14 Down |
| Tox2 | TOX high mobility group box family member 2 | 311615 | −1.22 Down |
| Map9 | Microtubule-associated protein 9 | 310544 | −1.26 Down |
| RGD1309108 | Similar to hypothetical protein FLJ23554 | 315578 | −1.55 Down |
| C, Exclusively expressed genes in Ma group
| |||
|---|---|---|---|
| Gene symbol | Description | Gene ID | Fold-change |
| Defb3 | β-defensin 3 | 641623 | 3.52 Up |
| RT1-Da | RT1 class II, locus Da | 294269 | 2.60 Up |
| RT1-Ba | RT1 class II, locus Ba | 309621 | 2.59 Up |
| Pxmp4 | Peroxisomal membrane protein 4 | 282634 | 2.46 Up |
| RT1-Bb | RT1 class II, locus Bb | 309622 | 2.21 Up |
| Ccl11 | Chemokine (C-C motif) ligand 11 | 29397 | 2.08 Up |
| Tmem252 | Transmembrane protein 252 | 361744 | 2.07 Up |
| Aurkb | Aurora kinase B | 114592 | 1.99 Up |
| Cd74 | Cd74 molecule, major histocompatibility complex, class II invariant chain | 25599 | 1.89 Up |
| Birc5 | Baculoviral IAP repeat-containing 5 | 64041 | 1.81 Up |
| Lmcd1 | LIM and cysteine-rich domains 1 | 494021 | 1.80 Up |
| Fam111a | Family with sequence similarity 111, member A | 499322 | 1.79 Up |
| RSA-14-44 | RSA-14-44 protein | 297173 | 1.77 Up |
| Kif11 | Kinesin family member 11 | 171304 | 1.70 Up |
| Vdac1 | Voltage-dependent anion channel 1 | 83529 | 1.70 Up |
| Hmgn3 | High mobility group nucleosomal binding domain 3 | 113990 | 1.69 Up |
| Nalcn | Sodium leak channel, non-selective | 266760 | 1.69 Up |
| Tnfrsf14 | Tumor necrosis factor receptor superfamily, member 14 | 366518 | 1.67 Up |
| Pex11a | Peroxisomal biogenesis factor 11 α | 85249 | 1.61 Up |
| RGD1564664 | Similar to LOC387763 protein | 499839 | 1.61 Up |
| LOC100911604 | CD99 antigen-like protein 2-like, similar to MIC2L1 | 500410 | −1.14 Down |
| Serpinb1b | Serine (or cysteine) peptidase inhibitor, clade B member 1b, leukocyte elastase inhibitor A-like | 306891 | −1.15 Down |
| Mgll | Monoglyceride lipase | 29254 | −1.15 Down |
| Lrtomt | Leucine rich transmembrane and 0-methyltransferase domain containing | 308868 | −1.16 Down |
| Tas2r145 | Taste receptor, type 2, member 145 | 100363053 | −1.18 Down |
| Kcnip3 | Kv channel interacting protein 3, calsenilin | 65199 | −1.19 Down |
| Fbll1 | Fibrillarin-like 1 | 363563 | −1.19 Down |
| LOC100910054 | NF-κ-B-repressing factor-like | 100910054 | −1.20 Down |
| Ttll1 | Tubulin tyrosine ligase-like family, member 1 | 362969 | −1.21 Down |
| Negr1 | Neuronal growth regulator 1 | 59318 | −1.22 Down |
| Ttll11 | Tubulin tyrosine ligase-like family, member 11 | 689746 | −1.24 Down |
| Mgam | Maltase-glucoamylase | 312272 | −1.25 Down |
| Apba1 | Amyloid β(A4) precursor protein-binding, family A, member 1 | 83589 | −1.26 Down |
| Hoxb5 | Homeo box B5 | 497987 | −1.26 Down |
| Zfp238 | Zinc finger protein 238 | 64619 | −1.27 Down |
| Ddx6 | DEAD (Asp-Glu-Ala-Asp) box helicase 6 | 500988 | −1.29 Down |
| LOC310902 | Similar to Alcohol dehydrogenase 1A (alcohol dehydrogenase α subunit) | 310902 | −1.30 Down |
| Fgf13 | Fibroblast growth factor 13 | 84488 | −1.32 Down |
| Tnnc2 | Troponin C type 2 (fast) | 296369 | −1.33 Down |
| Pdyn | Prodynorphin | 29190 | −1.43 Down |
log2 (Ratio) Mo, morphine (morphine infusion/vehicle injection/morphine challenge); Up, upregulated; Down, downregulated.
Reversed gene expression panel between MO/C and MOMa/C groups
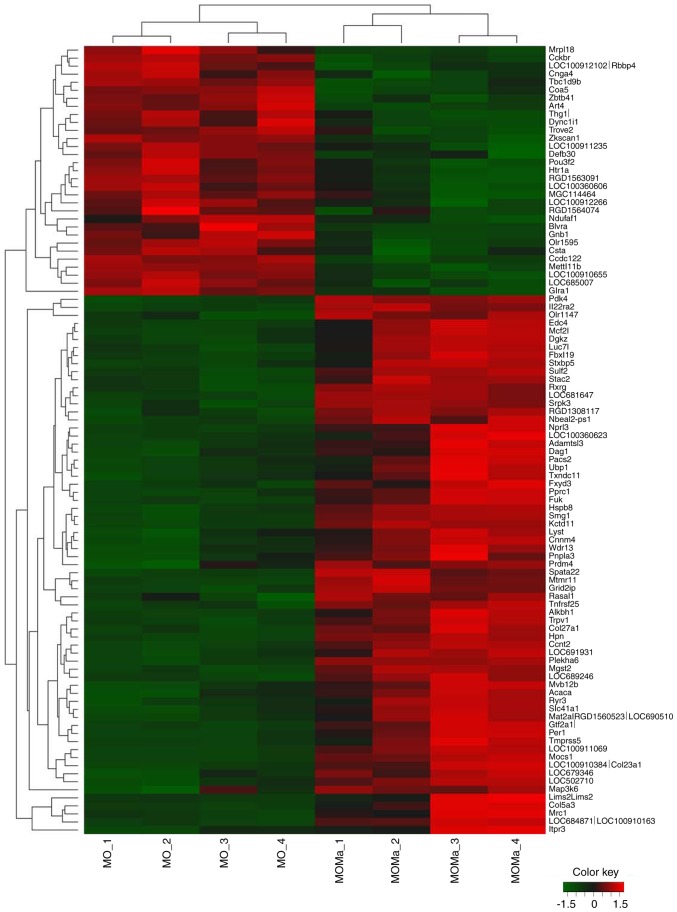
In order to clarify which genes were altered by melatonin treatment in morphine-tolerant rats, genes from the microarray data with inverted gene expression profiles between MO/C and MOMa/C groups were selected. Hierarchical clustering analysis was performed to construct a reversed gene expression heatmap between the MO/C and MOMa/C groups (Fig. 3). Genes were selected from the microarray data with the criteria of log2 ratio ≥1 or ≤−1 and P<0.05, and the expression of the genes was reversed between the MO/C and MOMa/C groups. The constructed panel according to the heatmap of the reversed genes was listed in Table V. The panel with inverted gene expression may be used to investigate potential pathways derived by melatonin treatment.
Figure 3.
Hierarchical clustering analysis of genes with expression completely inverted between the MO and MOMa group. Upregulated genes are indicated in red, and downregulated genes are presented in green. The signal intensity values of each sample were transformed to log2 values and subjected to hierarchical clustering using standard correlation. MO, morphine (morphine infusion/vehicle injection/morphine challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge).
Table V.
Genes with inverted expressions between Mo and MOMa group
| A, Genes with upregulated expression in the MO group but downregulated expression in the MOMa group
| ||||
|---|---|---|---|---|
| Gene symbol | Description | Gene ID | Fold MO | Fold MOMA |
| Glra1 | Glycine receptor, α1 | 25674 | 2.10 | 0.65 |
| Olr1595 | Olfactory receptor 1595 | 304990 | 1.70 | 0.66 |
| Ndufaf1 | NADH dehydrogenase (ubiquinone) complex I, assembly factor 1 | 296086 | 1.42 | 0.72 |
| RGD1563091 | Similar to OEF2 | 500011 | 1.40 | 0.79 |
| Gnb1 | Guanine nucleotide binding protein (G protein), βpolypeptide 1 | 24400 | 1.40 | 0.76 |
| Ccdc122 | Coiled-coil domain containing 122 | 100360752 | 1.39 | 0.61 |
| Csta | Cystatin A (stefin A) | 288067 | 1.35 | 0.70 |
| Blvra | Biliverdin reductase A | 116599 | 1.33 | 0.70 |
| MGC114464 | Similar to expressed sequence AI836003 | 500925 | 1.32 | 0.75 |
| LOC100910655 | Paralemmin-2-like | 100910655 | 1.31 | 0.64 |
| Dync1i1 | Dynein cytoplasmic 1 intermediate chain 1 | 29564 | 1.31 | 0.79 |
| LOC685007 | Similar to unc-93 homolog A | 685007 | 1.30 | 0.68 |
| Cckbr | Cholecystokinin B receptor | 25706 | 1.29 | 0.78 |
| Art4 | ADP-ribosyltransferase 4 | 312806 | 1.29 | 0.72 |
| Zbtb41 | Cytochrome C oxidase assembly factor 5 | 503252 | 1.28 | 0.75 |
| Cnga4 | Cyclic nucleotide gated channel α4 | 85258 | 1.28 | 0.78 |
| Thg1l | tRNA-histidine guanylyltransferase 1-like (S. cerevisiae) | 303067 | 1.26 | 0.82 |
| Mettl11b | Methyltransferase like 11B | 289167 | 1.25 | 0.68 |
| LOC100911235 | Mediator of RNA polymerase II transcription subunit 7-like | 100911235 | 1.25 | 0.81 |
| Htr1a | 5-hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | 24473 | 1.24 | 0.75 |
| Pou3f2 | POU class 3 homeobox 2 | 29588 | 1.24 | 0.77 |
| B, Genes with downregulated expression in the MO group but upregulated expression in the MOMa group
| ||||
|---|---|---|---|---|
| Gene symbol | Description | Gene ID | Fold MO | Fold MOMA |
| Itpr3 | Inositol 1,4,5-trisphosphate receptor, type 3 | 25679 | 0.59 | 1.82 |
| Mocs1 | Molybdenum cofactor synthesis 1 | 301221 | 0.69 | 1.66 |
| Col5a3 | Collagen, type V, α3 | 60379 | 0.73 | 1.66 |
| Mrc1 | Mannose receptor, C type 1 | 291327 | 0.66 | 1.65 |
| Lims2 | LIM and senescent cell antigen like domains 2 | 361303 | 0.65 | 1.63 |
| Gtf2a1l | General transcription factor IIA, 1-like | 316711 | 0.76 | 1.58 |
| Olr1147 | Olfactory receptor 1147 | 300408 | 0.60 | 1.55 |
| Ccnt2 | Cyclin T2 | 304758 | 0.82 | 1.51 |
| Map3k6 | Mitogen-activated protein kinase kinase kinase 6 | 313022 | 0.64 | 1.45 |
| Tnfrsf25 | Tumor necrosis factor receptor superfamily, member 25 | 500592 | 0.71 | 1.43 |
| Slc41a1 | Solute carrier family 41, member 1 | 363985 | 0.83 | 1.43 |
| Mvb12b | Multivesicular body subunit 12B | 362118 | 0.80 | 1.42 |
| Acaca | Acetyl-CoA carboxylase α | 60581 | 0.77 | 1.38 |
| Ryr3 | Ryanodine receptor 3 | 170546 | 0.77 | 1.38 |
| Col27a1 | Collagen, type XXVII, α1 | 298101 | 0.83 | 1.36 |
| Rasal1 | RAS protein activator like 1 (GAP1 like) | 360814 | 0.72 | 1.36 |
| Rasal1 | RAS protein activator like 1 (GAP1 like) | 360814 | 0.72 | 1.36 |
| Mat2a | Methionine adenosyltransferase II | 690510 | 0.79 | 1.29 |
| er1 | Period circadian clock 1 | 287422 | 0.63 | 1.25 |
| Dgkz | Diacylglycerol kinase ζ | 81821 | 0.77 | 1.24 |
Mo, morphine (morphine infusion/vehicle injection/morphine challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge).
PANTHER pathway mapping and RT-qPCR analysis
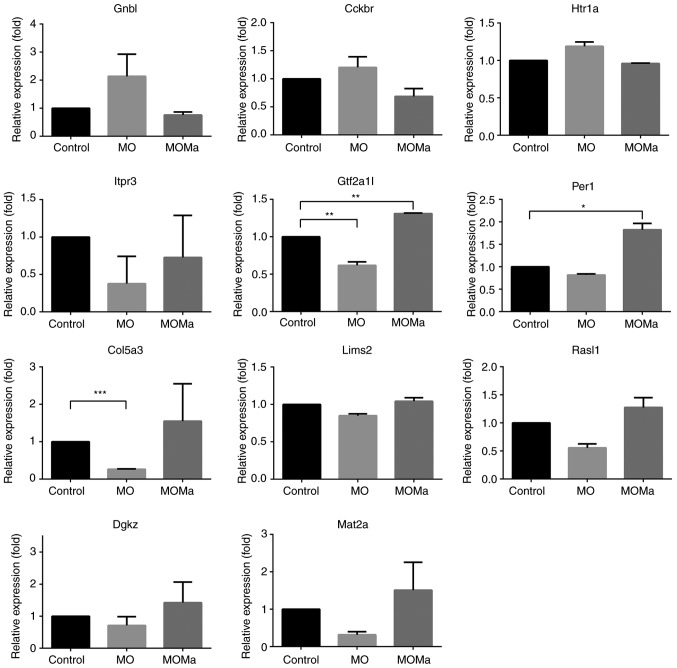
The genes listed in Table V-A and -B were used for enrichment analysis by the PANTHER algorithm provided by the GO Consortium. The PANTHER pathway mapped 4 out of 29 genes for the genes listed in Table V-A and 10 out of 66 genes for the genes listed in Table V-B. The PANTHER-mapped pathways and associated genes are listed in Table VI-A and -B. Guanine nucleotide binding protein β polypeptide 1 (Gnb1) was identified to participate in numerous cellular functions, including neuron-associated functions, including glutamatergic, cholinergic, GABAergic, dopaminergic, serotonergic and sympathetic neuron functions. Furthermore, Gnb1 has also been reported to participate in other pathways, including histamine H1 and H2 receptors and several hormone receptor signals. Gnb1 was upregulated 1.4-fold in the MO group and downregulated 1.3-fold in the MOMA group; this result implies the potential of a melatonin-mediated pathway via the repression of Gβ expression and signaling. On the other hand, the genes mapped in Table VI-B mainly participated in cell proliferation and migration in addition to cytoskeleton reconstruction. For example, a gene named inositol 1,4,5-trisphosphate receptor, type 3 (Itpr3) was downregulated 1.7-fold in the MO group but was upregulated 1.8-fold in the MOMA group. A number of pathways associated with Itpr3, including inflammatory, cell proliferation and migration, G protein mediated and vaso-relaxation pathways, were suggested. The genes mentioned in Table VI-A and VI-B were selected and their gene expressions were verified using RT-qPCR (Fig. 4). All the genes selected here demonstrated an accordance with the expression trends of the microarray results. The results indicate the potential signaling pathways of melatonin in the rat spinal cord for the restoration of cell proliferation and migration through cytoskeleton reconstruction.
Table VI.
PANTHER pathway mapped cellular functions of four selected genes from Table V-A and -B.
| A, PANTHER pathway mapped cellular functions of four selected genes from Table V-A
| ||
|---|---|---|
| Gene name | Cellular functions | Pathways |
| Guanine nucleotide binding protein, β polypeptide 1 (Gnb1) | Neuron | |
| Pain_Relief_anagelsia | Opioid proopiomelanocortin pathway | |
| Pain_Relief_anagelsia | Opioid proenkephalin pathway | |
| Pain_Relief_anagelsia | Enkephalin release | |
| Pain_Relief_anagelsia | Cortocotropin releasing factor receptor signaling pathway | |
| Pain_Relief_anagelsia | Opioid prodynorphin pathway | |
| Glutamertergic | Metabotropic glutamate receptor group II pathway | |
| Glutamertergic | Heterotrimeric G-protein signaling pathway-rod outer segment phototransduction | |
| Glutamertergic | Metabotropic glutamate receptor group III pathway | |
| Cholinergic | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |
| Cholinergic | β1 adrenergic receptor signaling pathway | |
| Cholinergic | Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |
| GABAergic | GABA-B_receptor_II_signaling | |
| GABAergic | Endogenous_cannabinoid_signaling | |
| Dopaminergic | Dopamine receptor mediated signaling pathway | |
| Serotonergic | 5HT2 type receptor mediated signaling pathway | |
| Serotonergic | 5HT4 type receptor mediated signaling pathway | |
| Depolarization | Nicotine pharmacodynamics pathway | |
| Sympathetic | β3 adrenergic receptor signaling pathway | |
| G protein mediated pathway | β2 adrenergic receptor signaling pathway | |
| Inflammation | ||
| G protein mediated pathway | Histamine H1 receptor mediated signaling pathway | |
| G protein mediated pathway | Histamine H2 receptor mediated signaling pathway | |
| G protein mediated pathway | Heterotrimeric G-protein signaling pathway-Gq a and Go α mediated pathway | |
| G protein mediated pathway | Thyrotropin-releasing hormone receptor signaling pathway | |
| Others | ||
| Signaling pathway | Gonadotropin releasing hormone receptor pathway | |
| Signaling pathway | PI3 kinase pathway | |
| Signaling pathway | Wnt signaling pathway | |
| Angiogenesis | Angiotensin II-stimulated signaling through G proteins and β-arrestin | |
| Muscle contraction | Oxytocin receptor mediated signaling pathway | |
| Cell migration | CCKR signaling pathway | |
| Cholecystokinin B receptor (Cckbr) | Cell migration | CCKR signaling pathway |
| 5-hydroxytryptamine (serotonin) receptor 1A | Neuron_Serotonergic | 5HT1 type receptor mediated signaling pathway |
| G protein-coupled (Htr1a) | G protein mediated pathway | Heterotrimeric G-protein signaling pathway-Gi α and Gs α mediated pathway |
| Dynein cytoplasmic 1 intermediate chain 1 (Dync1i1) | Neuron | Huntington disease |
| B, PANTHER pathway mapped cellular functions of four selected genes from Table V-B
| ||
|---|---|---|
| Gene name | Cellular functions | Pathways |
| Inositol 1,4,5-trisphosphate receptor type 3 (Itpr3) | Inflammation and immunity | Inflammation mediated by chemokine and cytokine signaling pathway |
| Histamine H1 receptor mediated signaling pathwayB cell activation | ||
| Cell proliferation and migration | Gonadotropin releasing hormone receptor pathway | |
| Wnt signaling pathway | ||
| Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||
| PDGF signaling pathway | ||
| G protein mediated pathway | Angiotensin II-stimulated signaling through | |
| G proteins and β-arrestin | ||
| Heterotrimeric G-protein signaling pathway-Gq α and Go α mediated pathway | ||
| Vaso relaxation | Endothelin signaling pathway | |
| RAS protein activator like 1 (Rasal1) | Cell proliferation and migration | FGF signaling pathway |
| EGF receptor signaling pathway | ||
| PDGF signaling pathway | ||
| General transcription factor subunit 1-like (Gtf2a1l) | Transcription regulation | Transcription regulation by bZIP transcription 2A |
| General transcription regulation | ||
| Period circadian protein | Biochemical oscillator | Circadian clock system |
| homolog 1 (Per1) | Cell proliferation and migration | Gonadotropin releasing hormone receptor pathway |
| S-adenosylmethionine synthase isoform type-2 (Mat2a) | Enzyme activity | S adenosyl methionine biosynthesis |
| Diacylglycerol kinase ζ (Dgkz) | Cell proliferation and migration | Gonadotropin releasing hormone receptor pathway |
| α4 type V collagen (Col5a3) | Cytoskeleton | Integrin signaling pathway |
| Collagen α-1(XXVII) chain (Col27a1) | Cytoskeleton | Integrin signaling pathway |
| LIM and senescent cell antigen-like-containing domain protein (Lims2) | Cytoskeleton | Integrin signaling pathway |
| Mitogen-activated protein kinase kinase kinase 6 (Map3k6) | Cell proliferation and migration | FGF signaling pathway |
Figure 4.
Expressions of genes of interests determined by reverse transcription-quantitative polymerase chain reaction. All expression levels were normalized using GAPDH expression. *P<0.05, **P<0.01 and ***P<0.001 with comparison shown by lines. MO, morphine (morphine infusion/vehicle injection/morphine challenge); MOMa, (morphine infusion/melatonin injection/morphine challenge); Gnb1, guanine nucleotide binding protein β polypeptide 1; Cckbr, cholecystokinin B receptor; Htr1a, 5-hydroxytryptamine receptor 1A; Itpr3, inositol 1,4,5-trisphosphate receptor type 3; Gtf2a1, general transcription factor IIA subunit 1; Per1, period circadian regulator 1; Col5a3, collagen type V α 3 chain; Lims2, LIM zinc finger domain containing 2; RasI1, RAS protein activator like 1, Dgkz, diaglycerol kinase ζ; Mat2a, methionine adenosyltransferase 2A.
Discussion
By using microarray analysis of rat spinal cords from different treatment groups, the gene expression alterations in different conditions were identified. Panels of gene expression with upregulation in the MO group but downregulation in the MOMa group and also inverted gene expression profiles between the MO/C and MOMa/C groups were constructed. Among them, a number of notable genes were identified following PANTHER pathway mapping. For example, Gnb1 is one of the three subunits of heterotrimeric guanine nucleotide binding proteins (G proteins), which integrate signals between G protein coupled receptors (GPCR) (28). GPCR signaling is initiated when a ligand-bound receptor activates heterotrimeric G proteins on the inner leaflet of the plasma membrane by catalyzing the exchange of GDP for GTP on G protein α subunit (Gα), causing it to release the Gβγ subunits. The GTP-bound Gα and free Gβγ subunits transmit the signal by engaging intracellular effector molecules until GTP is hydrolysed and the β subunits are recoupled to the α subunit to terminate signal transduction receptors and effectors (29). According to a study by Klein et al (30), Gnb1 belongs to a group of genes that is night/day differentially expressed in the pineal body; this result implies the potential involvement of Gnb1 in melatonin treatment. Furthermore, β-arrestin-2 mediated desensitization of the µ-opioid receptor is involved in morphine tolerance (31,32). In the present data, Gnb1 was upregulated in the MO group but downregulated by melatonin treatment; this result indicates the potential of a role of melatonin in the activation and desensitization of GPCR.
Another gene, Itpr3, was downregulated in the MO group but upregulated in the MOMA group. It was also produced following PANTHER pathway mapping. Itpr3 is the receptor for inositol 1,4,5-trisphosphate (IP3), which mediates the release of intracellular calcium (33). Following IP3 binding, Itpr3 permits calcium flow out of the endoplasmic reticulum (34) and results in the activation of transient receptor potential cation channel subfamily M member 5, which results in membrane depolarization (35). In the case of melatonin treatment with Itpr3 upregulation, it was speculated that depolarization in certain nerve cells in the spinal cord may participate in the melatonin-derived attenuation of antinociceptive morphine tolerance.
In the present model, long-term morphine administration did not affect opioid receptor expression potentially due to the alteration of signal transduction and receptor-G protein coupling. It has been demonstrated that the downregulation of opioid receptors following chronic agonist exposure induces tolerance (36,37). However, a controversial report did not observe the downregulation of opioid receptors in tolerant animals (38). On the other hand, studies suggest that β-arrestin-2 (Arrb2) binding causes OPR desensitization, and OPR endocytosis and recycling are required for receptor resensitization. This result suggests the potential involvement of Arrb2 with morphine tolerance (31). However, in the present data, the expression of Arrb2 between the groups was similar; the results did not support the potential involvement of Arrb2 with morphine tolerance. Combining the previous discussion with the present data, it was postulated that the expression changes of opioid receptor and Arrb2 are not mandatory for morphine tolerance mechanisms.
There are limitations in descriptive microarray studies. The first limitation is that sequences in the microarray will be refined in newer databases and will result in different outcomes. Secondly, the associations between mRNAs may be different between mice and humans (39). The third limitation is that the gene expression detected by microarrays is descriptive and may not reflect protein expression and subsequent post-transcriptional modifications (40). However, identifying changes in gene expression in tissues with a high-throughput approach remains a good option as it can be performed in one experiment. Even though the present study uses descriptive microarray analysis, a panel of genes that are specifically expressed in morphine-tolerant animals with or without melatonin treatment was produced. As it is impossible to collect the spinal cord from patients, therefore future studies will use drug databases to identify drugs which target the genes of interest in the present study and use the drugs in the same rat models as in the present study to assess the dosage and efficacy of the drug in the treatment of relieving the morphine tolerance. Following that, the drugs with efficacy in the rat model will be used in humans. Next, patients who are under chronic treatment and with morphine tolerance may be recruited to assess the efficacy of the drug in order to ascertain the results of the present and provide novel treatment methods. From the present microarray analysis, novel insight into the molecular profiles associated with morphine tolerance and the effects of melatonin was provided. The present study offers a foundation for future specific hypotheses testing on potential therapeutic targets derived from melatonin treatment in patients with long-term morphine exposure.
Acknowledgments
The authors would like to thank Dr. Chih-Cheng Chien for his advice about the research.
Funding
The present study was supported by research grants from Ministry of Science and Technology (grant no. 105-231 4-B-281-003-MY2) and Cathay General Hospital (grant no. CGH-MR-A10518) in Taiwan.
Authors’ contributions
YCC and RYT wrote the manuscript, were responsible for conception and design, data analysis and interpretation. YTS performed the microarray data analysis and interpretation. IJC was in charge of the animal experiments. TYT and YYM performed the RT-qPCR analysis and interpretation. CSW was responsible for conception and design, financial support, administrative support, final approval of manuscript.
Ethics approval and consent to participate
The use of rats in this study adhered to the Guiding Principles in the Care and Use of Animals of the American Physiology Society and was ethically approved by the National Defense Medical Center Animal Care and Use Committee (Taipei, Taiwan).
Patient consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen WW, Zhang X, Huang WJ. Pain control by melatonin: Physiological and pharmacological effects. Exp Ther Med. 2016;12:1963–1968. doi: 10.3892/etm.2016.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasternak GW. When it comes to opiates, just say NO. J Clin Invest. 2007;117:3185–3187. doi: 10.1172/JCI34035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Günther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S. Targeting multiple opioid receptors-improved analgesics with reduced side effects. Br J Pharmacol. 2018;175:2857–2868. doi: 10.1111/bph.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang CS, Tsang SF, Yang JC. Effects of melatonin, morphine and diazepam on formalin-induced nociception in mice. Life Sci. 2001;68:943–951. doi: 10.1016/S0024-3205(00)00996-6. [DOI] [PubMed] [Google Scholar]
- 7.Lin SH, Huang YN, Kao JH, Tien LT, Tsai RY, Wong CS. Melatonin reverses morphine tolerance by inhibiting microglia activation and HSP27 expression. Life Sci. 2016;152:38–43. doi: 10.1016/j.lfs.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Song L, Wu C, Zuo Y. Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: Role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol. 2015;15:12. doi: 10.1186/1471-2253-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin W, Chun W, Ling L, Wei W. Role of melatonin in the prevention of morphine-induced hyperalgesia and spinal glial activation in rats: Protein kinase C pathway involved. Int J Neurosci. 2012;122:154–163. doi: 10.3109/00207454.2011.635828. [DOI] [PubMed] [Google Scholar]
- 10.Feng YM, Jia YF, Su LY, Wang D, Lv L, Xu L, Yao YG. Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy. 2013;9:1395–1406. doi: 10.4161/auto.25468. [DOI] [PubMed] [Google Scholar]
- 11.Yahyavi-Firouz-Abadi N, Tahsili-Fahadan P, Riazi K, Ghahremani MH, Dehpour AR. Melatonin enhances the anti-convulsant and proconvulsant effects of morphine in mice: Role for nitric oxide signaling pathway. Epilepsy Res. 2007;75:138–144. doi: 10.1016/j.eplepsyres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Raghavendra V, Kulkarni SK. Reversal of morphine tolerance and dependence by melatonin: Possible role of central and peripheral benzodiazepine receptors. Brain Res. 1999;834:178–181. doi: 10.1016/S0006-8993(99)01520-6. [DOI] [PubMed] [Google Scholar]
- 13.Garmabi B, Vousooghi N, Vosough M, Yoonessi A, Bakhtazad A, Zarrindast MR. Effect of circadian rhythm disturbance on morphine preference and addiction in male rats: Involvement of period genes and dopamine D1 receptor. Neuroscience. 2016;322:104–114. doi: 10.1016/j.neuroscience.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Liang X, Wang R, Song L. Role of endogenous melatoninergic system in development of hyperalgesia and tolerance induced by chronic morphine administration in rats. Brain Res Bull. 2017;135:105–112. doi: 10.1016/j.brainresbull.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Chou KY, Tsai RY, Tsai WY, Wu CT, Yeh CC, Cherng CH, Wong CS. Ultra-low dose (+)-naloxone restores the thermal threshold of morphine tolerant rats. J Formos Med Assoc. 2013;112:795–800. doi: 10.1016/j.jfma.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Guiding principles in the care and use of animals of the American physiology society
- 17.Grossman ML, Basbaum AI, Fields HL. Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms) J Comp Neurol. 1982;206:9–16. doi: 10.1002/cne.902060103. [DOI] [PubMed] [Google Scholar]
- 18.Tsai RY, Chou KY, Shen CH, Chien CC, Tsai WY, Huang YN, Tao PL, Lin YS, Wong CS. Resveratrol regulates N-methyl-D-aspartate receptor expression and suppresses neuroinflammation in morphine-tolerant rats. Anesth Analg. 2012;115:944–952. doi: 10.1213/ANE.0b013e31825da0fb. [DOI] [PubMed] [Google Scholar]
- 19.Pirooznia M, Nagarajan V, Deng Y. GeneVenn-A web application for comparing gene lists using Venn diagrams. Bioinformation. 2007;1:420–422. doi: 10.6026/97320630001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Kelly R, Fang H, Tong W. ArrayTrack: A free FDA bioinformatics tool to support emerging biomedical research-an update. Hum Genomics. 2010;4:428–434. doi: 10.1186/1479-7364-4-6-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saldanha AJ. Java Treeview-extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 22.Gene ontology Enrichment analysis website.
- 23.Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 41 (Database Issue) 2013:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience. 2016;338:160–182. doi: 10.1016/j.neuroscience.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Kwon HS, Johnson TV, Joe MK, Abu-Asab M, Zhang J, Chan CC, Tomarev SI. Myocilin mediates myelination in the peripheral nervous System through ErbB2/3 signaling. J Biol Chem. 2013;288:26357–26371. doi: 10.1074/jbc.M112.446138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontenille L, Rouquier S, Lutfalla G, Giorgi D. Microtubule-associated protein 9 (Map9/Asap) is required for the early steps of zebrafish development. Cell Cycle. 2014;13:1101–1114. doi: 10.4161/cc.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 29.Thal DM, Vuckovic Z, Draper-Joyce CJ, Liang YL, Glukhova A, Christopoulos A, Sexton PM. Recent advances in the determination of G protein-coupled receptor structures. Curr Opin Struct Biol. 2018;51:28–34. doi: 10.1016/j.sbi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Klein DC, Bailey MJ, Carter DA, Kim JS, Shi Q, Ho AK, Chik CL, Gaildrat P, Morin F, Ganguly S, et al. Pineal function: Impact of microarray analysis. Mol Cell Endocrinol. 2010;314:170–183. doi: 10.1016/j.mce.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 32.Zuo Z. The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg. 2005;101:728–734. doi: 10.1213/01.ANE.0000160588.32007.AD. table of contents. [DOI] [PubMed] [Google Scholar]
- 33.Nagaleekar VK, Diehl SA, Juncadella I, Charland C, Muthusamy N, Eaton S, Haynes L, Garrett-Sinha LA, Anguita J, Rincón M. IP3 receptor-mediated Ca2+ release in naive CD4 T cells dictates their cytokine program. J Immunol. 2008;181:8315–8322. doi: 10.4049/jimmunol.181.12.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor CW, Tovey SC. IP(3) receptors: Toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stafford K, Gomes AB, Shen J, Yoburn BC. mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/S0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- 38.Polastron J, Meunier JC, Jauzac P. Chronic morphine induces tolerance and desensitization of mu-opioid receptor but not down-regulation in rabbit. Eur J Pharmacol. 1994;266:139–146. doi: 10.1016/0922-4106(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Liu H, Jiao Y, Wang E, Clark SH, Postlethwaite AE, Gu W, Chen H. Differences between mice and humans in regulation and the molecular network of collagen, type III, alpha-1 at the gene expression level: Obstacles that translational research must overcome. Int J Mol Sci. 2015;16:15031–15056. doi: 10.3390/ijms160715031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brodsky AN, Caldwell M, Harcum SW. Glycosylation and post-translational modification gene expression analysis by DNA microarrays for cultured mammalian cells. Methods. 2012;56:408–417. doi: 10.1016/j.ymeth.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.