Abstract
In our previous study, the 4-aminobutyrate aminotransferase (ABAT) gene was screened and selected as a target gene that may affect the prognosis of myelo-dysplastic syndrome (MDS). The present study aimed to determine the prognostic value of ABAT in 152 patients with MDS, 29 patients with acute myeloid leukemia (AML) and 40 controls, by detecting the expression and methylation levels of the ABAT gene. In patients with MDS, the expression levels of ABAT were significantly reduced compared with in the controls (P<0.0001), and the degree of DNA methylation was increased in MDS subjects (P<0.0001). Age, hemoglobin level, marrow blasts, International Prognostic Scoring System karyotype, and the expression and methylation levels of ABAT were associated with overall survival (OS), as determined by univariate analysis. Multivariate analysis revealed that older age, higher marrow blasts and higher methylation percentage were independent risk factors for OS. In addition, a functional study demonstrated that ABAT gene silencing increased cell apoptosis and blocked the G1/S phase in SKM-1 and THP-1 human leukemia cells. A γ-aminobutyrate aminotransferase inhibitor also blocked the G1/S phase; however, it had no effect on cell apoptosis. In conclusion, the present study demonstrated that ABAT methylation served an essential role in the progression of MDS and therefore may be considered an indicator of poor prognosis for hematological malignancies.
Keywords: ABAT gene, DNA methylation, overall survival, myelodysplastic syndrome
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous hema-topoietic malignancy that is characterized by ineffective hematopoiesis, and is accompanied by abnormal maturation and dysplasia in one or more blood cell lineages (1). The pathogenesis of MDS is currently unclear; however, it has been reported to be associated with gene mutations, immune dysregulation, chromosomal abnormalities, epigenetic abnormalities and other factors. DNA methylation is an important research topic in epigenetics, which has been demonstrated to be involved in the development and progression of MDS. In addition, it is a biomarker for the diagnosis, treatment and survival of patients with MDS (2-5).
In our previous study, six genes [4-aminobutyrate aminotransferase (ABAT), dual adaptor of phosphotyrosine and 3-phosphoinositides 1, Fas-associated via death domain, LRR-binding FLII-interacting protein 1, phospholipase B domain-containing 1 and sphingomyelin phosphodiesterase 3] were screened out through genome-wide DNA methylation profiling and gene expression microarray in four patients with MDS and four controls (4). In addition, significant alterations have been observed in the expression levels of ABAT, as compared with other genes, after leukemia cell lines are treated with decitabine, which is a hypomethylating agent (6). Therefore, this gene was selected as a target gene.
ABAT encodes γ-aminobutyrate aminotransferase (GABAT), which participates in catabolism of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (7). Recently, Besse et al reported an essential role for ABAT in the mitochondrial nucleoside salvage pathway, which may facilitate the conversion of deoxyribonucleoside diphosphate into deoxyribonucleoside triphosphate, and maintain the function of the mitochondrial membrane (8). Furthermore, alterations in the expression levels of ABAT take part in the development of breast cancer and hepatocellular carcinoma (9-11). However, to the best of our knowledge, no studies have focused on the pathogenic mechanisms and prognostic value of ABAT in patients with MDS.
In the present study, the expression and methylation levels of ABAT were detected in 152 patients with MDS, 29 patients with acute myeloid leukemia (AML) and 40 controls using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and methylation-sensitive high resolution melting (MS-HRM), respectively. The expression and methylation levels of ABAT were found to be associated with overall survival (OS) in patients with MDS. In addition, cell viability, cell apoptosis and cell cycle progression were analyzed in leukemia cell lines with ABAT gene silencing or in cell lines treated with a GABAT inhibitor, in order to demonstrate the role of ABAT in the pathogenesis of MDS.
Materials and methods
Patients and bone marrow samples
Bone marrow samples were extracted from 152 adult patients with MDS and 29 patients with AML. All patients were diagnosed according to the 2008 World Health Organization (WHO) criteria (12). Of the patients with MDS, 92 were men and 60 were women, with a median age of 56 years (range, 18-86 years). A total of 84 patients had refractory cytopenia with multilineage dysplasia (RCMD), 8 had RCMD with ring sideroblasts (RCMD-RS), 17 had refractory anemia with an excess of blasts type 1 (RAEB-1), 29 had RAEB-2, five had refractory anemia (RA), two had refractory anemia with ring sideroblasts (RARS), six had an unclassifiable form of MDS (MDS-U) and one had 5q-syndrome. Of the patients with AML, 16 were diagnosed with acute myelomonocytic leukemia, six with acute monocytic leukemia (AML-M5) and seven with AML with mixed-lineage leukemia rearrangements. The prognostic score for each patient with MDS was calculated using the International Prognostic Scoring System (IPSS) (13). All subjects with MDS were recruited on January 1, 2008 by the Sino-U.S. Shanghai Leukemia Cooperative Group (School of Public Health, Fudan University, Shanghai, China), and were followed up until they succumbed to the disease or until the last follow-up date (December 30, 2015). Two subjects did not undergo chromosomal examination due to examination failure. Finally, 69 subjects succumbed and 14 subjects progressed to AML. In addition, bone marrow samples from 40 subjects without hematological malignancies were obtained between December 30, 2015 and January 1, 2018, and were analyzed as controls. The control subjects were diagnosed as having immune thrombocytopenia by bone marrow smears and chromosomal examinations. Subsequently, a 2-year follow-up period ensured that these controls did not develop MDS or other clonal disorders. No statistical difference was found with regards to age and sex among the subjects with MDS and AML and the controls. The present study was approved by the Ethics Committee of Huashan Hospital, Fudan University (approval no. KY2015-269; Shanghai, China), and informed consent was obtained from each participant.
RNA isolation and RT-qPCR
The mononuclear cells were harvested by bone marrow aspiration and were isolated by centrifugation (600 × g for 20 min at room temperature) in lymphocyte separation medium (Ficoll® Paque Plus; GE Healthcare, Chicago, IL, USA). Total RNA was isolated from bone marrow mononuclear cells using the QIAamp RNA Blood Mini kit (Qiagen GmbH, Hilden, Germany). TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to isolate total RNA from the cultured leukemia cells after culture medium was removed and the cells were washed with pre-cooled PBS (4°C) three times. The purity of RNA was assessed by measuring the optical density (OD) 260/280 ratio using a NanoDrop spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). The RNA samples were then reverse transcribed into cDNA using Takara PrimeScript RT Master Mix (Takara Bio, Inc., Otsu, Japan). The 10-µl reaction mixture comprised 2 µl 5X PrimeScript RT Master Mix, 500 ng RNA and RNase-Free distilled H2O, which was used to ensure the total reached 10 µl. Subsequently, the mixture was amplified at 37°C for 15 min, 85°C for 5 sec and was stored at 4°C for further use.
For RT-qPCR, cDNA samples were amplified using an ABI Prism 7500 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with Takara SYBR Premix Ex Taq PCR reagents (Takara Bio, Inc.). A 20-µl reaction mixture containing 10 µl SYBR-Green premix, 0.4 µl each primer (10 µM), 0.4 µl ROX Reference Dye II, 2 µl cDNA and 6.8 µl DEPC-treated water was amplified at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 34 sec. The PCR primer sequences for ABAT were as follows: Forward, 5′-TTTTCTGTCTCCTCCACCTGTC-3′ and reverse, 5′-CTGGCT GGGTTCATCCTAAG-3′, which resulted in amplification of a 113-bp PCR band. The GAPDH pr i mer sequences were as follows: For wa rd, 5′-TGGATGAAGTTGGTGGTGAG-3′ and reverse, 5′-CAGCATCAGGAGTGGACAGA-3′, which resulted in amplification of an 87-bp PCR band. The relative abundance of ABAT mRNA was normalized to the levels of GAPDH mRNA using the 2−ΔΔCq (fold change) method (14).
DNA isolation and MS-HRM
Total DNA was isolated from bone marrow mononuclear cells using QIAamp DNA Blood Mini kit (Qiagen GmbH), according to the manufacturer’s protocol. DNA quality and purity were assessed using the aforementioned methods, and DNA samples were stored at −20°C for further use.
For MS-HRM, DNA samples from all patients, and human methylated and nonmethylated DNA standards (Zymo Research Corp., Irvine, CA, USA) were modified using the EZ DNA Methylation-Gold kit (Zymo Research Corp.), according to the manufacturer’s protocol. In addition, the human methylated and nonmethylated DNA standards were mixed at a ratio of 1:9, 1:3, 1:1 and 3:1 to perform DNA methylation modification. All modified DNA samples were amplified using the C1000 Touch Thermal Cycler (Bio-Rad Laboratories, Inc.) with Takara Premix Taq Hot Start Version (Takara Bio, Inc.). A 25-µl reaction mixture containing 12.5 µl Premix Taq Hot Start Version, 1 µl each primer (10 µM), 1 µl modified DNA and 9.5 µl DEPC-treated water was amplified at 95°C for 2 min, followed by 40 cycles at 95°C for 30 sec, 59°C for 30 sec and 72°C for 30 sec, and an extension step at 72°C for 5 min. Subsequently, the amplified product was added to 0.9 µl SYTO 9 Green Fluorescent Nucleic Acid Stain (Invitrogen; Thermo Fisher Scientific, Inc.) and reacted at 94°C for 60 sec and 40°C for 60 sec. Data were collected between 75°C and 85°C using Rotor Gene 6000 (Qiagen GmbH). The methylation primers for ABAT were as follows: Forward, 5′-GTAAGAAGGGTTGGTAGGGTTTT-3′ and reverse, 5′-ACCATTTACACCCTCAAAACTACA-3′, which resulted in amplification of a 183-bp PCR band.
Cell culture and short hairpin (sh)RNA knockdown of ABAT
In the present study, SKM-1 and THP-1 leukemia cell lines were used. The SKM-1 cell line is derived from a patient with leukemia transformed from MDS; therefore, this cell line has similar characteristics to MDS. The THP-1 cell line is derived from a patient with AML-M5. Mutations in the ABAT gene were detected in both cell lines used in this study. The ABAT gene mutations detected in the SKM-1 cell line were as follows: 309C>T, V103V; 984C>A, V328V and 167G>A, Q56R. The ABAT gene mutations in the THP-1 cell line were: 984C>A, V328V and 167G>A, Q56R. The mutation sites have no influence on enzyme activity; therefore, these two cell lines were used in the present functional study. The human myeloid leukemia cell line THP-1 was obtained from the Chinese Academy of Sciences (Beijing, China), and the SKM-1 cell line was purchased from Health Science Research Resources Bank (Sennan, Japan). All cells were cultured in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2.
shRNA sequences that targeted the 3′ untranslated region of ABAT were designed and cloned into pGMLV-SC5 lentiviral vectors (Genomeditech, Shanghai, China). The oligonucleotide sequences were: Sh1-ABAT, 5′-GCGGGAGGACCTGCTAAATAA-3′; Sh2-ABAT, 5′-GCTGGAGACGTGCATGATTAA-3′; and Sh3-ABAT, 5′-GGTGACAAATCCATTCGTTTC-3′. SKM-1 and THP-1 cells (1×105) were infected with three independent shRNA lentiviruses and a scramble lentivirus (negative control, NC; Genomeditech) at a multiplicity of infection of 80 at 37°C; cells without lentiviral infection were considered normal control cells. Infection efficiency was evaluated by flow cytometry, according to the percentage of green fluorescent protein (GFP)-positive cells, and RT-qPCR and western blotting after 72 h. Puromycin (cat. no. P8230; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used to improve the percentage of GFP-positive cells at 72 h.
Drug treatment
For drug treatment, the two cell lines were cultured in complete medium supplemented with 0, 100, 200 and 400 µmol/l (±)-vigabatrin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), which is a specific inhibitor of the GABAT enzyme; the medium was replaced by new complete medium containing (±)-vigabatrin every 24 h. After 10 days, the cells were harvested for further use (15).
GABAT enzymatic activity assay
GABAT enzymatic activity was detected using the GABAT assay kit (cat. no. E-134; Biomedical Research Service Center, University at Buffalo, Buffalo, NY, USA), according to a previous report (15). Briefly, this assay is based on sequential GABA transamination reaction and glutamate dehydrogenase reaction, which couples the reduction of iodonitrotetrazolium to iodonitrotetrazolium-formazan (ε = 18 mM−1cm−1 at 492 nm). Reactions were terminated by adding 3% acetic acid (cat. no. A6283; Sigma-Aldrich; Merck KGaA) and OD was measured at 492 nm using a plate reader (Thermo Fisher Scientific, Inc.). A mean of the readings was obtained, and control well readings were subtracted from sample well readings (ΔOD). GABAT activity was calculated using the following formula: GABAT activity [µmol/(l•min)] = (ΔOD × 1,000 × 150 µl)/(60 min × 0.6 cm × 18 × 5 µl) = ΔOD × 46.3, where 150 µl is the total reaction volume, 0.6 cm is the light path in the 96-well plate, and 5 µl is the volume of the sample in each well.
Cell viability assay
Cell viability was measured using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Rockville, MD, USA) according to the manufacturer’s protocol. Briefly, cells treated with the GABAT inhibitor, cells infected with shRNA lentiviruses, NC cells and normal control cells were seeded in a 96-well plate at 5×103 cells/well. The plates were then incubated for 24, 48 and 72 h at 37°C in a humidified incubator containing 5% CO2. Subsequently, 10 µl CCK-8 reagent was added to each well. After 4 h at 37°C, absorbance was measured at 450 nm. Three independent experiments were performed.
Measurement of cell apoptosis and cell cycle distribution using flow cytometry
The two cell lines (1×105 cells) infected with lentiviral vectors or treated with a GABAT inhibitor were harvested and washed with PBS. Cell apoptosis was assessed using Phycoerythrin (PE) Annexin V Apoptosis Detection kit I (cat. no. 559763) and Fluorescein Isothiocyanate (FITC) Annexin V Apoptosis Detection kit I (cat. no. 556547) (both from BD Biosciences, Franklin Lakes, NJ, USA), respectively. Briefly, the cells were harvested and the supernatant was discarded; subsequently, cells were suspended in PBS containing 3% bovine serum albumin (BSA; cat. no. A8020; Beijing Solarbio Science & Technology Co., Ltd.) and were adjusted to 1×106 cells/ml. A 100-µl aliquot was labeled with 5 µl PE and 5 µl 7-aminoactinomycin D (7-AAD) for cells transfected with lentiviruses, or with 5 µl FITC and 5 µl propidium iodide (PI) for cells treated with drugs, and cells were incubated at room temperature for 25 min. Flow cytometry was performed immediately after incubation. Data acquisition and analysis were performed using the BD FACSCanto flow cytometer (BD Biosciences) with FCS Express 3.0 software (De Novo Software, Glendale, CA, USA) and FlowJo 7.6 software (FlowJo LLC, Ashland, OR, USA). Normal controls were used to set voltage for flow cytometry. Cells that were Annexin V-positive and 7-amino-actinomycin D (7-AAD)/propidium iodide (PI)-negative were the early apoptotic fraction, whereas cells that were double positive were the late apoptotic fraction.
For cell cycle analysis, the aforementioned leukemia cells (1×105 cells) were harvested, washed twice with ice-cold PBS, and fixed with 75% ethanol at −20°C overnight. Prior to analysis, the fixed cells were washed twice with ice-cold PBS and suspended in 300 µl PI for 15 min in the dark at 4°C. The data were acquired using the BD FACSCanto flow cytometer with FCS Express 3.0 software. Data were analyzed using ModFit LT software (version 3.2; Verity Software House, Inc., Topsham, ME, USA).
Western blot analysis
For western blot analysis, total protein was extracted from the cultured cells using protein lysis buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.) and was quantified using a bicinchoninic acid assay kit (Beyotime Biotechnology, Haimen, China), with BSA (Beijing Solarbio Science & Technology Co., Ltd.) as a standard. Proteins (20 µg) were fractionated by 10% SDS-PAGE and were transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked in 5% non-fat dry milk at room temperature for 2 h and were immunostained overnight at 4°C using antibodies against ABAT (cat. no. ab216465, 1:1,000; Abcam, Cambridge, MA, USA), cyclin D1 (cat. no. 2978S, 1:1,000), cyclin D3 (cat. no. 2936S, 1:1,000), cyclin-dependent kinase (CDK)4 (cat. no. 12790S, 1:1,000), CDK6 (cat. no. 13331S, 1:1,000), p16INK4a (cat. no. 80772S, 1:1,000), p21Waf1/Cip1 (cat. no. 2947S, 1:1,000), caspase-3 (cat. no. 9665T, 1:1,000), cleaved caspase-3 (cat. no. 9664T, 1:1,000), B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax; cat. no. 2772T, 1:1,000), Bcl-2 (cat. no. 4223T, 1:1,000), GAPDH (cat. no. 2118S, 1:1,000) and β-actin (cat. no. 8457S, 1:1,000) (Cell Signaling Technology, Danvers, MA, USA). GAPDH and β-actin antibodies were used as loading controls. After washing with Tris-buffered saline containing 0.1% Tween, the membranes were incubated with the following secondary antibodies: Anti-rabbit immunoglobulin G (IgG) (cat. no. 7074P2, 1:5,000; Cell Signaling Technology, Inc.) and anti-mouse IgG (cat. no. ab6728, 1:5,000; Abcam) at room temperature for 1 h. The protein bands were visualized using the enhanced chemiluminescence system (Beyotime Institute of Biotechnology). Densitometric analysis of the protein bands was conducted using ImageJ software (ImageJ 1.48v; National Institutes of Health, Bethesda, MD, USA). The ratio was calculated by comparing the gray value of proteins to GAPDH or β-actin.
Statistical analysis
All statistical analyses were performed using Stata version 14.0 software (StataCorp LP, College Station, TX, USA); comparisons between two groups were analyzed by independent t-test when data conformed to normal distribution, if not, a non-parametric Kruskal-Wallis test was performed. The 95% confidence interval (CI) of the expression and methylation levels of ABAT in the 40 control samples was defined as the normal level. Values that were below or above the confidence limit were considered low or high expression/methylation. The independent t-test was used to compare the levels between two groups. One-way analysis of variance followed by Bonferroni post hoc test was used for multiple comparisons. χ2 tests or Fisher’s exact tests were used for prognostic analysis.
OS was assessed from the day of diagnosis until death due to any cause, or until the last follow-up date (December 30, 2015). Subjects who succumbed prior to leukemia evolution were censored at the time of death. Kaplan-Meier curves were generated to assess the association of gene expression and methylation with survival, and the log-rank test was performed to analyze the association between different clinical indicators and survival. The Cox regression model was used for multivariate analysis, in order to identify independent prognostic factors affecting OS. P<0.05 was considered to indicate a statistically significant difference.
Results
mRNA expression levels of ABAT in patients with MDS and AML, and control individuals
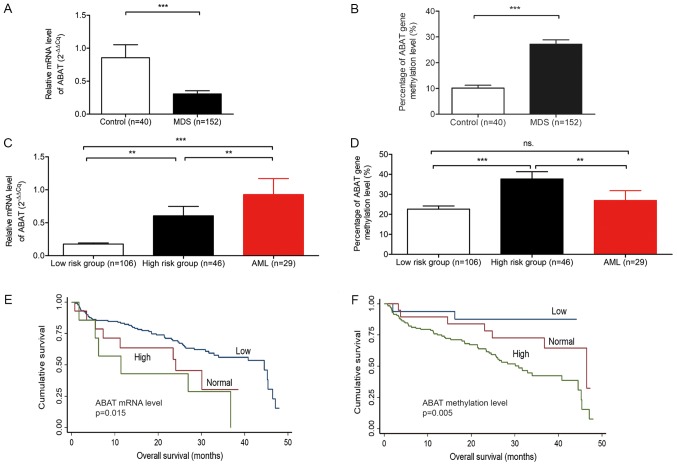
The mRNA expression and methylation levels of ABAT were detected in samples obtained from 152 patients with MDS, 29 patients with AML and 40 controls individuals (Fig. 1A-D). The average expression level in the 152 patients with MDS was 0.31 (0.21-0.40), which was significantly lower compared with in the controls (P<0.0001; Fig. 1A and Table I). When the number of bone marrow blast cells was increased, the mRNA expression levels of ABAT were also upregulated (Table I). According to the WHO subtypes of MDS, patients with high-risk subtypes (i.e. RAEB-1 and RAEB-2) exhibited high expression levels compared with low-risk subtypes (i.e., RA, RARS, RCMD, RCMD-RS, 5q-syndrome and MDS-U) (P=0.09; Fig. 1C and Table I). The low-risk and high-risk subgroups of patients with MDS exhibited lower expression levels than patients with AML (P=0.0001 and P=0.0039, respectively).
Figure 1.
Expression and methylation levels of ABAT in patients with MDS and AML, and control individuals. (A) Reverse transcription-quantitative polymerase chain reaction analysis of relative mRNA expression levels of ABAT in bone marrow samples from patients with MDS and controls. (B) Methylation-sensitive high resolution melting analysis of methylation degree of ABAT in patients with MDS and controls. (C) Relative mRNA expression levels of ABAT were analyzed in low-risk subjects, high-risk subjects and subjects with AML. (D) Methylation levels of ABAT in the three groups. (E) Overall survival curve of patients with MDS with various mRNA expression levels of ABAT. (F) Overall survival curve of patients with MDS with various degrees of ABAT methylation. The low-risk MDS group included refractory anemia, refractory anemia with ring sideroblasts, 5q-syndrome, unclassified MDS, RCMD and RCMD with ring sideroblasts subgroups; the high-risk MDS group included RAEB-1 and RAEB-2. **P<0.01 and ***P<0.001. ABAT, 4-aminobutyrate aminotransferase; MDS, myelodysplastic syndrome; RAEB; refractory anemia with excess of blasts; RCMD, refractory cytopenia with multilineage dysplasia.
Table I.
ABAT mRNA expression and methylation in patients with MDS and AML, and control individuals.
| Variable | No. of patients | Relative ABAT mRNA levels (95% CI) | P-value | Degree of ABAT methylation, % (95% CI) | P-value |
|---|---|---|---|---|---|
| Group | <0.0001 | <0.0001 | |||
| Controls | 40 | 0.86 (0.46-1.25) | 10.18 (7.98-12.39) | ||
| MDS patients | 152 | 0.31 (0.21-0.40) | 27.23 (23.95-30.51) | ||
| AML patients | 29 | 0.93 (0.43-1.43) | 26.99 (16.94-37.06) | ||
| Age (years) | 0.542 | 0.947 | |||
| <60 | 83 | 0.31 (0.18-0.45) | 26.98 (22.38-31.57) | ||
| ≥60 | 69 | 0.29 (0.15-0.43) | 27.52 (22.75-32.30) | ||
| Sex | 0.386 | 0.788 | |||
| Male | 92 | 0.34 (0.21-0.47) | 26.74 (22.76-30.72) | ||
| Female | 60 | 0.25 (0.11-0.39) | 27.97 (22.19-33.76) | ||
| WBCs (×109/l) | 0.472 | 0.575 | |||
| <4 | 129 | 0.33 (0.21-0.44) | 26.15 (22.94-29.37) | ||
| ≥4 | 23 | 0.19 (0.13-0.25) | 33.25 (20.68-45.82) | ||
| Neutrophils (×109/l) | 0.289 | 0.286 | |||
| <1.5 | 114 | 0.32 (0.20-0.45) | 25.83 (22.34-29.32) | ||
| ≥1.5 | 38 | 0.25 (0.15-0.36) | 31.41 (23.33-39.49) | ||
| Hemoglobin (g/dl) | 0.336 | 0.477 | |||
| <9 | 115 | 0.34 (0.22-0.46) | 27.04 (23.59-30.49) | ||
| ≥9 | 37 | 0.20 (0.12-0.28) | 27.80 (19.30-36.31) | ||
| PLTs (×109/l) | 0.146 | 0.896 | |||
| <100 | 112 | 0.34 (0.21-0.46) | 26.79 (23.10-30.48) | ||
| ≥100 | 40 | 0.21 (0.11-0.31) | 28.45 (21.22-36.68) | ||
| WHO classification | 0.004 | <0.0001 | |||
| RA, RARS, RCMD, RCMD-RS, MDS-U, 5q-syndrome | 106 | 0.18 (0.15-0.21) | 22.62 (19.47-25.77) | ||
| RAEB-1, RAEB-2 | 46 | 0.41 (0.31-0.89) | 37.84 (30.48-45.20) | ||
| BM blasts (%) | 0.049 | <0.0001 | |||
| <5 | 106 | 0.18 (0.15-0.21) | 22.68 (19.54-25.82) | ||
| 5-9 | 21 | 0.44 (0.16-0.71) | 46.26 (33.73-58.79) | ||
| 10-19 | 25 | 0.72 (0.22-1.21) | 30.86 (22.29-39.42) | ||
| IPSS karyotypea | 0.654 | 0.056 | |||
| Good | 104 | 0.27 (0.17-0.36) | 25.27 (21.36-29.16) | ||
| Intermediate | 29 | 0.38 (0.13-0.64) | 28.48 (20.87-36.08) | ||
| Poor | 17 | 0.39 (0.07-0.85) | 36.14 (24.87-47.41) | ||
| IPSS cytopeniasb | 0.358 | 0.807 | |||
| 0/1 | 19 | 0.27 (0.07-0.48) | 29.14 (16.98-41.30) | ||
| 2/3 | 133 | 0.31 (0.21-0.41) | 26.96 (23.56-30.35) | ||
| IPSS risk groupc | 0.007 | 0.004 | |||
| Low | 13 | 0.20 (0.06-0.35) | 23.45(9.06-37.83) | ||
| INT-1 | 96 | 0.20 (0.14-0.26) | 23.72 (20.34-27.11) | ||
| INT-2 | 26 | 0.44 (0.11-0.77) | 34.75 (25.13-44.37) | ||
| High | 15 | 0.81 (0.15-1.47) | 38.73 (24.08-53.39) |
An independent t-test was used to compare two groups. One-way analysis of variance was used for multiple group comparisons. ABAT, 4-aminobutyrate aminotransferase; AML, acute myeloid leukemia; BM, bone marrow; INT, intermediate; IPSS; International Prognostic Scoring System; MDS, myelodysplastic syndrome; MDS-U, unclassified MDS; PLTs, platelets; RA, refractory anemia; RAEB-1; refractory anemia with excess of blasts type 1; RAEB-2, refractory anemia with excess of blasts type 2; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, RCMD with ring sideroblasts; WBCs, white blood cells; WHO, World Health Organization.
IPSS karyotype: Good, normal, 5q-, 20q-, -Y; intermediate, not good or poor; poor, complex aberration or chromosome 7 abnormalities.
IPSS cytopenias: Hemoglobin concentration <9 g/dl, absolute neutrophil count <1.8×109/l, PLT count <100×109/l.
IPSS risk groups: Low, 0; INT-1, 0.5-1.0; INT-2, 1.5-2.0; high, ≥2.5, according to IPSS score.
MS-HRM of patients with MDS and AML, and control individuals
The fluorescence intensities of 0, 10, 25, 50, 75 and 100% methylated DNA standards were detected (data not shown) and the fluorescence intensity of nonmethylated DNA standards was subtracted to determine differential fluorescence intensity. A standard curve was established, with y = 0.622x + 0.326, R2=0.998 (data not shown). Subsequently, the fluorescence intensities of DNA methylation of 152 patients with MDS, 29 patients with AML and 40 controls were detected, in order to calculate the percentage of ABAT methylation according to the standard curve. The percentage of ABAT gene methylation was higher in subjects with MDS than in controls (P=0.0001) (Table I and Fig. 1B). The high-risk subgroup of MDS patients exhibited a higher degree of methylation compared with the low-risk subgroup (P<0.0001). No difference was determined between patients with AML and low-risk subjects (P=0.417; Fig. 1D).
Association of the expression and methylation levels of ABAT with the prognosis of patients with MDS
Univariate analysis revealed that age, hemoglobin levels, WHO classification, marrow blast percentage, IPSS karyotype, IPSS cytopenias, IPSS risk group, and the expression and methylation levels of ABAT were significant associated with OS in patients with MDS (Table II). The normal expression levels of ABAT (95% CI, 0.46-1.25) and the normal methylation percentage of ABAT (95% CI, 7.98-12.39) were defined in the 40 control samples (Table I). Values below the lower confidence limit were considered the low-expression and low-methylation group; values above the high confidence limit were considered the high-expression and high-methylation group; the other values were considered normal. Therefore, according to this classification, subjects with MDS were categorized into 14 normal-, 131 low- and 7 high-expression patients. In terms of ABAT gene methylation, subjects were categorized into 16 low-, 19 normal- and 117 high-methylation subjects (Table II).
Table II.
Univariate analysis of prognostic factors for patients with MDS.
| Variable | No. of patients | Cases of mortality | P-value |
|---|---|---|---|
| Age (years) | 0.004 | ||
| <60 | 83 | 31 | |
| ≥60 | 69 | 38 | |
| Sex | 0.996 | ||
| Male | 92 | 41 | |
| Female | 60 | 28 | |
| WBCs (×109/l) | 0.804 | ||
| <4 | 129 | 59 | |
| ≥4 | 23 | 10 | |
| Neutrophils (×109/l) | 0.310 | ||
| <1.5 | 114 | 56 | |
| ≥1.5 | 38 | 13 | |
| Hemoglobin (g/dl) | 0.005 | ||
| <9 | 115 | 59 | |
| ≥9 | 37 | 10 | |
| PLTs (×109/l) | 0.070 | ||
| <100 | 112 | 56 | |
| ≥100 | 40 | 13 | |
| WHO classification | <0.001 | ||
| RA, RARS, RCMD, RCMD-RS, 5q-syndrome, MDS-U | 106 | 33 | |
| RAEB-1, RAEB-2 | 46 | 36 | |
| BM blasts (%) | <0.001 | ||
| <5 | 106 | 32 | |
| 5-9 | 21 | 17 | |
| 10-19 | 25 | 20 | |
| IPSS karyotypea | 0.007 | ||
| Good | 104 | 41 | |
| Intermediate | 29 | 15 | |
| Poor | 17 | 12 | |
| IPSS cytopeniasb | 0.045 | ||
| 0/1 | 19 | 4 | |
| 2/3 | 133 | 65 | |
| IPSS risk groupsc | <0.001 | ||
| Low | 13 | 2 | |
| INT-1 | 96 | 33 | |
| INT-2 | 26 | 21 | |
| High | 15 | 12 | |
| ABAT mRNA expression | 0.015 | ||
| Low (<0.46) | 131 | 55 | |
| Normal (0.46-1.25) | 14 | 8 | |
| High (>1.25) | 7 | 6 | |
| ABAT methylation degree (%) | 0.005 | ||
| Low (<7.98) | 16 | 2 | |
| Normal (7.98-12.39) | 19 | 7 | |
| High (>12.39) | 117 | 60 |
For evaluation of prognostic factors, χ2 tests or Fisher’s exact tests were used. ABAT, 4-aminobutyrate aminotransferase; BM, bone marrow; CI, confidence interval; INT, intermediate; IPSS; International Prognostic Scoring System; MDS, myelodysplastic syndrome; MDS-U, unclassified MDS; PLTs, platelets; RA, refractory anemia; RAEB-1; refractory anemia with excess of blasts type 1; RAEB-2, refractory anemia with excess of blasts type 2; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, RCMD with ring sideroblasts; WBCs, white blood cells; WHO, World Health Organization.
IPSS karyotype: Good, normal, 5q-, 20q-, -Y; intermediate, not good or poor; poor, complex aberration or chromosome 7 abnormalities.
IPSS cytopenias: Hemoglobin concentration <9 g/dl, absolute neutrophil count <1.8×109/l, PLT count <100×109/l.
IPSS risk groups: Low, 0; INT-1, 0.5-1.0; INT-2, 1.5-2.0; high, ≥2.5, according to IPSS score.
Subjects with normal and high ABAT mRNA expression had a shorter OS compared with those with low expression (P=0.015; Fig. 1E). The median survival time of subjects with high expression was 17.88 months. Furthermore, when the degree of methylation increased, OS decreased (P=0.005; Fig. 1F).
The multivariate Cox regression analysis demonstrated that old age, high blast count and high methylation had a strong impact on the OS of patients with MDS (hazard ratios=2.51, 2.26 and 1.93, respectively); therefore, these were considered independent risk factors for the survival of patients with MDS (Table III). Although ABAT mRNA expression had prognostic value in the univariate analysis, the multivariate analysis revealed that the expression of ABAT was not an independent factor affecting OS (P=0.856).
Table III.
Multivariate analysis of prognostic factors in patients with myelodysplastic syndrome.
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Age (≥60 years) | 2.51 (1.48-4.25) | 0.001 |
| Hemoglobin (<9 g/dl) | 0.79 (0.36-1.74) | 0.573 |
| BM blasts (≥5%) | 2.26 (1.66-3.09) | <0.0001 |
| IPSS karyotype (poor) | 1.27 (0.91-1.78) | 0.145 |
| IPSS cytopenias (2/3) | 1.38 (0.89-2.16) | 0.148 |
| ABAT mRNA expression (high) | 1.04 (0.68-1.57) | 0.856 |
| Degree of ABAT methylation (high) | 1.93 (1.09-3.40) | 0.022 |
ABAT, 4-aminobutyrate aminotransferase; BM, bone marrow; CI, confidence interval; HR, hazard ratio; IPSS, International Prognostic Scoring System.
Effects of ABAT shRNA on SKM-1 and THP-1 cells
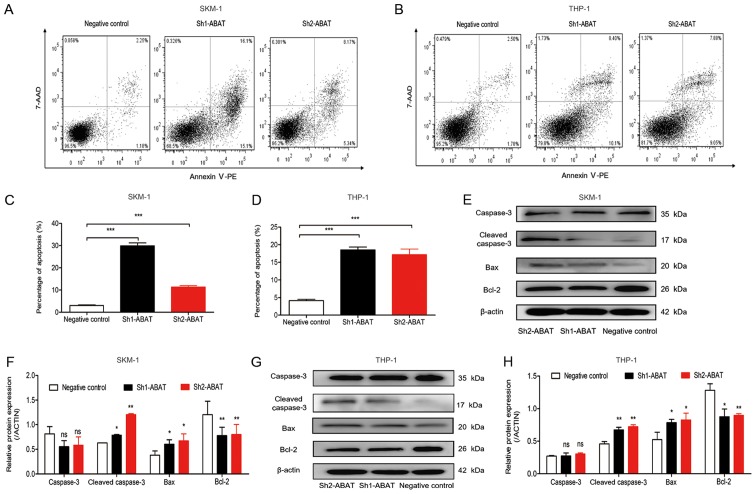
The mRNA and protein expression levels of ABAT were detected in the various cell groups (Fig. 2). There was no difference in ABAT expression between SKM-1 and THP-1 cell lines (data not shown). HL-60 is also a human leukemia cell line; however, due to its low expression of ABAT (data not shown), SKM-1 and THP-1 cells were used in this study. Three lentiviral vectors were constructed containing three different shRNA sequences (i.e. Sh1-ABAT, Sh2-ABAT and Sh3-ABAT), in order to explore the biological function of ABAT in leukemia cell lines. Following infection with the three vectors, the mRNA expression levels of ABAT were decreased by 90, 70 and 50% in SKM-1 cells compared with the NC group, respectively (Fig. 2A). In THP-1 cells, following infection with the three vectors, the mRNA expression levels of ABAT were decreased by 80, 60 and 40%, respectively (Fig. 2B). There was no difference between the normal control and negative control groups with regards to ABAT mRNA expression. The protein expression levels of ABAT were decreased by ~90, 80 and 50% (Fig. 2C and E) in the Sh1-ABAT, Sh2-ABAT and Sh3-ABAT SKM-1 cells, and by 60, 55 and 30% in the THP-1 cells (Fig. 2D and F) compared with the NC group, respectively. Since the Sh3-ABAT lentivirus exerted minor effects on the mRNA and protein expression levels of ABAT, Sh1-ABAT and Sh2-ABAT lentiviruses were used for further functional analysis.
Figure 2.
Expression levels of ABAT and relative enzyme activity of GABAT in SKM-1 and THP-1 cells. Relative mRNA expression levels of ABAT in (A) SKM-1 and (B) THP-1 cells 72 h post-infection, as determined by reverse transcription-quantitative polymerase chain reaction. Normal control cells were not infected with a lentiviral vector. Western blot analysis of (C) SKM-1 and (D) THP-1 cells in the normal control, NC and shRNA lentivirus groups. Gray value analysis of ABAT protein expression in (E) SKM-1 and (F) THP-1 cells infected with NC and shRNA lentiviruses. Relative enzyme activity of GABAT in (G) SKM-1 and (H) THP-1 cells with ABAT silencing. Relative activity of GABAT in (I) SKM-1 and (J) THP-1 cells treated with various concentrations of GABAT inhibitor. Data are presented as the means ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. the normal control or 0 µM group. ABAT, 4-aminobutyrate aminotransferase; GABAT, γ-aminobutyrate aminotransferase; NC, negative control; ns, not significant; sh/shRNA, short hairpin RNA.
Relative activity of GABAT in cells infected with shRNA lentiviruses and treated with a GABAT inhibitor
Relative GABAT activity was detected following infection of SKM-1 and THP-1 cells with shRNA lentiviruses. Relative GABAT activity was decreased by 65 and 40% in the Sh1-ABAT and Sh2-ABAT groups compared with in the NC group in SKM-1 cells (Fig. 2G), and by 35 and 40% in THP-1 cells (Fig. 2H), respectively. (±)-vigabatrin is a specific inhibitor of GABAT; therefore, relative activity of GABAT was also detected in cells following vigabatrin treatment. When the dose of vigabatrin was increased, GABAT activity was decreased to 70% in the 100 µM group, 60% in the 200 µM group and 55% in the 400 µM group compared with in the control group in SKM-1 cells (Fig. 2I). In THP-1 cells, relative GABAT activity was decreased to 70, 40 and 45% in the 100, 200 and 400 µM groups, respectively, compared with in the control group (Fig. 2J).
ABAT gene silencing and GABAT inhibition inhibits leukemia cell viability
SKM-1 and THP-1 cells were infected with shRNA lentiviruses and the effects of ABAT knockdown on viability were evaluated. Cells that were not infected with lentivirus or treated with the GABAT inhibitor were considered normal controls; all results were compared to the normal control group. The results revealed that when the expression levels of ABAT were decreased, cell viability was significantly inhibited (Fig. 3A and B). Furthermore, treatment with the GABAT inhibitor also inhibited the viability of SKM-1 and THP-1 cells (Fig. 3C and D). Taken together, these data indicated important roles for ABAT and GABAT in the pathogenesis of MDS.
Figure 3.
Viability of SKM-1 and THP-1 cells with ABAT gene silencing and GABAT inhibitor treatment. Viability of (A) SKM-1 and (B) THP-1 cells with ABAT gene silencing following puromycin selection at 24, 48 and 72 h. Viability of (C) SKM-1 and (D) THP-1 cells treated with various concentrations (0, 100, 200 and 400 µM) of the GABAT inhibitor. *P<0.05, **P<0.01 and ***P<0.001 vs. the normal control or 0 µM group. ABAT, 4-aminobutyrate aminotransferase; GABAT, γ-aminobutyrate aminotransferase; sh, short hairpin RNA.
Apoptosis of cells with ABAT gene silencing and following treatment with a GABAT inhibitor
Cell apoptosis is generally used as an indicator of alterations in biological behavior. Annexin V-PE/7-AAD and Annexin V/PI staining, followed by flow cytometry, were used to analyze apoptosis of cells infected with lentiviruses and treated with vigabatrin, in order to determine whether cell apoptosis was affected by ABAT gene silencing and GABAT inhibition. As shown in Fig. 4, the percentage of cell apoptosis increased following ABAT gene knockdown using Sh1-ABAT and Sh2-ABAT lentiviruses. The fraction of apoptotic cells was 29.07±0.21 and 11.40±0.57% in Sh1-ABAT- and Sh2-ABAT-infected SKM-1 cells, and 3.04±0.27% in the negative control group (Fig. 4A and C). The percentage of apoptotic cells was 18.53±0.81 (P<0.001) and 17.18±1.6% (P<0.001) in Sh1-ABAT- and Sh2-ABAT-infected THP-1 cells compared with in the negative control group (Fig. 4B and D). The expression levels of apoptosis-associated proteins, including cleaved caspase-3 and Bax were increased, whereas the expression levels of the anti-apoptotic protein Bcl-2 were decreased in SKM-1 (Fig. 4E and F) and THP-1 cells (Fig. 4G and H) in response to ABAT knockdown.
Figure 4.
Apoptosis analysis of SKM-1 and THP-1 cells following ABAT gene silencing using flow cytometry and western blotting. Apoptosis of (A) SKM-1 and (B) THP-1 cells with ABAT gene silencing, as determined by Annexin V-PE/7-AAD staining at 72 h. (C) Percentage of apoptotic (C) SKM-1 and (D) THP-1 cells following infection with negative control and shRNA lentiviruses. Western blot analysis of apoptosis-associated proteins in (E and F) SKM-1 and (G and H) THP-1 cells with ABAT gene silencing at 72 h, including caspase-3, cleaved caspase-3, Bax and Bcl-2. (F and H) Gray value analysis of apoptotic proteins was conducted. Data are presented as the means ±standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. negative control or as indicated. 7-AAD, 7-aminoactinomycin D; ABAT, 4-aminobutyrate aminotransferase; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; PE, phycoerythrin; sh/shRNA, short hairpin RNA.
Cell apoptosis was also detected following drug treatment. Treatment with a GABAT inhibitor had a minor impact on cell apoptosis. In SKM-1 cells, the percentage of apoptotic cells was 5.35±0.65, 5.50±0.46, 6.67±0.19 and 6.84±1.15% following treatment with 0, 100, 200 and 400 µM vigabatrin, respectively (data not shown). In THP-1 cells, the percentage of apoptotic cells was 4.97±0.82, 5.07±0.13, 5.80±0.94 and 5.49±1.2% following treatment with 0, 100, 200 and 400 µM vigabatrin, respectively; no statistically significant difference was detected between the groups (data not shown). In addition, the expression levels of apoptosis-associated proteins exhibited no difference between the control and drug-treated groups (data no shown).
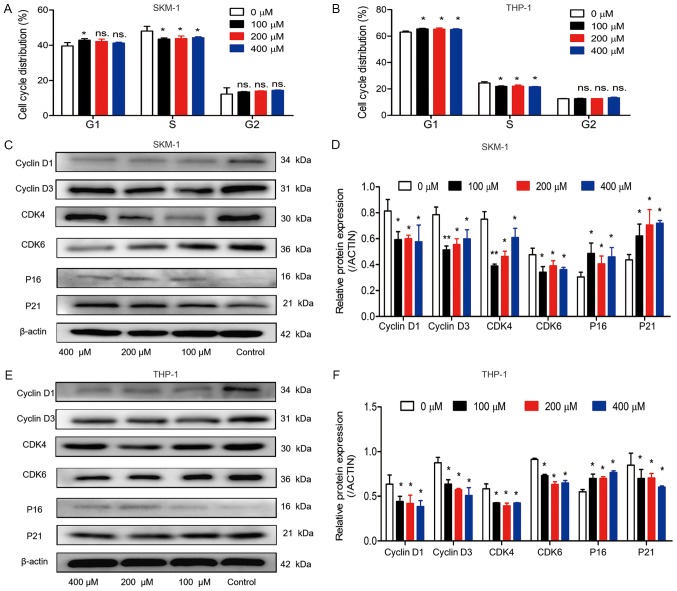
Effects of ABAT gene silencing and GABAT inhibition on cell cycle distribution
Cell cycle distribution was detected in the two cell lines, in order to identify whether the inhibitory effects of ABAT gene silencing and GABAT inhibition on growth could be explained by alterations in the cell cycle. In SKM-1 cells, following infection with Sh1-ABAT and Sh2-ABAT, the percentage of cells in G1 phase was increased to 53.09±4.69 (P<0.01) and 52.85±2.54% (P<0.01) compared with in the negative control group, and the percentage of cells in S phase was decreased to 34.28±0.29 and 34.89±0.22%, respectively (Fig. 5A). The same trend was observed in the THP-1 cells (Fig. 5B). There was no difference between the negative control and normal control groups. In these two cell lines, cell cycle-associated proteins were also detected. The protein expression levels of CDK6, CDK4, cyclin D1 and cyclin D3 were significantly downregulated, whereas the expression levels of the periodic inhibitory proteins, p16INK4a and p21Waf1/Cip1, were upregulated in SKM-1 cells in response to ABAT knockdown (Fig. 5C and D). Conversely, in THP-1 cells, the expression of p21Waf1/Cip1 was decreased (Fig. 5E and F).
Figure 5.
Cell cycle distribution and associated protein expression in SKM-1 and THP-1 cells following ABAT gene silencing. Cell cycle distribution of (A) SKM-1 and (B) THP-1 cells in the normal control, negative control and sh-ABAT groups. Expression levels of cell cycle proteins in (C and D) SKM-1 cells and (E and F) THP-1 cells with ABAT gene silencing, including cyclin D1, cyclin D3, CDK4, CDK6, p16 and p21. (D and F) Gray value analysis of cell cycle-associated proteins was conducted. *P<0.05, **P<0.01 and ***P<0.001 vs. the negative control group or normal control group. ABAT, 4-aminobutyrate aminotransferase; CDK, cyclin-dependent kinase; ns, not significant; sh, short hairpin RNA.
Cell cycle distribution was also measured following drug treatment. When SKM-1 and THP-1 cells were treated with 100, 200 and 400 µM vigabatrin for 10 days, the percentage of cells in G0/G1 phase was slightly increased (Fig. 6A and B). Furthermore, in SKM-1 cells, the percentage of cells in S phase was decreased from 50.28±5.82% to 43.51±1.12, 43.83±2.49 and 44.32±0.87% in response to 100, 200 and 400 µM vigabatrin (Fig. 6A). In THP-1 cells, the percentage of cells in S phase was decreased from 25.44±1.65% to 21.90±0.61, 22.09±1.44 and 21.61±0.30% in response to 100, 200 and 400 µM vigabatrin (Fig. 6B). The expression levels of cell cycle-associated proteins were also detected by western blotting. A similar trend was observed in cells treated with the GABAT inhibitor as in cells with ABAT gene knockdown (Fig. 6C-F).
Figure 6.
Cell cycle distribution and associated protein expression in SKM-1 and THP-1 cells treated with various concentrations of a γ-aminobutyrate aminotransferase inhibitor (0, 100, 200 and 400 µM). Cell cycle distribution in (A) SKM-1 and (B) THP-1 cells following treatment with various drug concentrations. Expression levels of cell cycle-associated proteins in (C and D) SKM-1 and (E and F) THP-1 cells following treatment with various drug concentrations. (D and F) Gray value analysis of cell cycle-associated proteins was conducted. Data are presented as the means ± standard deviation. *P<0.05 and **P<0.01 vs. 0 µM. ABAT, 4-aminobutyrate aminotransferase; CDK, cyclin-dependent kinase; ns, not significant.
Discussion
Alterations in DNA methylation have been implicated in the pathogenesis of MDS and AML, and methylation serves as an indicator for disease progression and treatment efficacy (5). Several genes have been reported to be highly methylated in patients with MDS, including fragile histidine triad, inhibitor of DNA binding 4, HLH protein, suppressor of cytokine signaling 1 and glutathione peroxidase 3. As the disease progresses, these genes exhibit a higher methylation degree in high-risk groups compared with in low-risk groups (16-20). In the present study, high-risk patients (RAEB-1 and RAEB-2) with MDS exhibited a higher degree of ABAT gene methylation compared with low-risk subjects. Furthermore, patients with high methylation of ABAT had a poor prognosis, which was in concordance with previous reports on other genes in patients with MDS (16,18,21,22). Notably, when the number of bone marrow blasts and the IPSS risk group increased, the degree of ABAT methylation also increased. Therefore, it may be concluded that increased methylation of ABAT could indicate progression in patients with MDS.
Chromosomal abnormalities and gene mutations are considered progression-associated drivers in patients with MDS (23). Several gene mutations, including in isocitrate dehydrogenase (NADP(+)) 1, cytosolic (IDH1), isocitrate dehydrogenase (NADP(+)) 2, mitochondrial (IDH2), tet methylcytosine dioxygenase 2, ASXL transcriptional regulator 1 and enhancer of zeste 2 polycomb repressive complex 2 subunit, have been revealed to induce epigenetic alterations in hematological malignancies (24). GABAT can catalyze the conversion of GABA into succinic semialdehyde (SSA). SSA is then reduced by SSA dehydrogenase to form succinic acid, which enters the tricarboxylic acid (TCA) cycle (25,26). Mutations in IDH1 and IDH2 genes, which serve an important role in the TCA cycle, have been reported to participate in the pathogenesis and progression of MDS (27); therefore, it was hypothesized that mutations in the ABAT gene may be involved in the pathogenesis of MDS, due to its relation to the TCA cycle. However, the frequency of ABAT mutations is very low; the mutation sites include 631C>T, 275G>A, 1433T>C, 659G>A, 454C>T and 888G>T (8,15,28). ABAT mutations can lead to inactivation of ABAT and elevated GABA concentrations. Patients with these mutations display symptoms, including severe psychomotor retardation, hypotonia, hyperreflexia, seizures, high-pitched cry, growth acceleration and early mortality (8). However, in the present study, the patients with MDS exhibited no such symptoms; therefore, it was hypothesized that these patients did not have ABAT mutations. Single-nucleotide polymorphisms (SNPs) of ABAT have been reported to be closely associated with numerous diseases, including autism, gastroesophageal reflux disease and affective disorder (29-31). Therefore, the existence of SNPs and their correlation with MDS may be worth exploring.
In the present study, it was suggested that altered expression levels of ABAT may participate in the pathogenesis of MDS; therefore, the expression levels of ABAT were detected in patients with MDS and AML, and control individuals. Control patients had immune thrombocytopenia, and were considered suitable controls for MDS. In terms of routine blood examination, MDS and immune thrombocytopenia both present with cytopenia; in addition, MDS is a hemopoietic clonal disease, whereas immune thrombocytopenia is not a clonal disease of the blood system. The results demonstrated that the mRNA expression levels of ABAT were reduced in patients with MDS compared with in the controls. The present findings indicated that reduced ABAT expression in patients with MDS may be associated with high methylation of the ABAT gene. Notably, as the number of bone marrow blasts increased, so did the expression levels of ABAT. Subjects in the high-risk group exhibited higher ABAT expression compared with in the low-risk group; therefore, it was suggested that high ABAT expression may be used to predict disease progression for patients with MDS. However, in this study, ABAT gene expression was higher in control patients compared with in patients with MDS. Using multivariate analysis, it was demonstrated that the expression of ABAT was not an independent factor for prognosis. Gene expression can be regulated at the DNA and RNA level; therefore, ABAT expression may not be a good predictor for disease progression. However, low expression levels of ABAT have been reported to be associated with poor prognosis in patients with breast cancer and hepatocellular carcinoma (9-11). Disease types and the complexity of disease pathogenesis may be important factors.
In patients with cancer, aberrant hypermethylation in the promoter region often results in reduced gene expression (1). In our previous study, it was hypothesized that ABAT gene hypermethylation led to reduced expression (6). In the present study, when analyzing the expression and methylation levels of ABAT in patients with MDS and AML, the same trend was observed with regards to methylation and mRNA expression. The results of a survival analysis indicated that higher ABAT methylation was associated with poor prognosis in patients. In multivariate analysis, ABAT gene methylation was revealed to be an independent indicator for disease progression. In addition, the methylation degree of ABAT was revealed to be an independent prognostic factor. In AML, an inverse correlation has been detected between gene expression (CCAAT enhancer binding protein α and CCAAT enhancer binding protein δ) and methylation (32). In addition to methylation, several other factors influence gene expression, including chromatin remodeling, transcriptional activation from a new transcription start site (33), intragenic DNA methylation (34) and abnormal histone acetylation (35). The exact mechanisms underlying gene regulation require further exploration in patients with MDS.
It has previously been demonstrated that conversion of GABA into SSA and glutamate is catalyzed by GABAT (26). GABAT-derived glutamate is then catabolized by glutamate dehydrogenase into α-ketoglutaric acid, which enters the TCA cycle (26). The ABAT gene also participates in the mitochondrial nucleoside salvage pathway. If ABAT gene expression is knocked down and relative activity of GABAT is decreased, DNA depletion of mitochondria occurs (8). Since ABAT is closely associated with the TCA cycle and mitochondrial nucleoside salvage pathway, and the methylation level of ABAT was considered a prognostic indicator for patients with MDS, the biological function of ABAT in leukemia cells was determined, in order to evaluate its involvement in the pathogenesis of MDS.
In the present study, when ABAT was knocked down, the percentage of apoptosis was significantly increased, and the expression levels of apoptosis-associated proteins, including cleaved caspase-3 and Bax, were upregulated. Simultaneously, cell cycle distribution was detected by flow cytometry, and it was revealed that cell cycle progression was blocked in G1/S phase in response to ABAT knockdown. Furthermore, the expression levels of associated proteins, including CDK4, CDK6, cyclin D1 and cyclin D3, were decreased. In tumor cells, the CDK/cyclin D-retinoblastoma protein kinase complex regulates G1/S transition, and decreased CDK/cyclin D activity can result from the low expression of D-type cyclins or CDK4/6 (36). In the present study, knockdown of ABAT gene expression led to reduced CDK/cyclin D protein expression and blockage of G1/S phase, thus indicating that the ABAT gene may be crucial in the pathogenesis of MDS. Notably, two groups of CDK inhibitors (CDKIs) regulate CDK/cyclin D activity: The INK4 family (notably p16INK4a) and Cip/Kip family (p21Waf1/Cip1, p27 and p57) (37). High expression of CDKIs is often associated with inhibition of CDK expression. Correspondingly, cells with low expression of CDK4 and CDK6 frequently exhibit upregulation of antiperiodic proteins p16INK4a and p21Waf1/Cip1 (38). In THP-1 cells, p16 expression was similar to that in SKM-1 cells; however, the expression of p21Waf1/Cip1 protein was downregulated in response to ABAT knockdown. p21Waf1/Cip1, as a CDKI, is a characterized modulator of p53-induced cell cycle arrest, which is recognized as an important tumor suppressor gene; inactivation of p53 can lead to reduced expression of p21Waf1/Cip1 (39). In THP-1 cells, p53 activity should be determined following ABAT knockdown. When these cells were treated with the GABAT inhibitor vigabatrin, cell cycle progression was also blocked at the G1/S phase; however, the inhibitor had no effect on cell apoptosis. The present study hypothesized that the role of GABAT in MDS pathogenesis did not work only through enzyme activity. The mechanism in patients with MDS and leukemia cell lines requires further exploration.
In conclusion, the present study revealed that ABAT was highly methylated in patients with MDS. The mRNA expression levels and degree of methylation of ABAT in patients with MDS was dynamically altered with progression of disease. The age of subjects with MDS, hemoglobin levels, WHO classification, marrow blast levels, IPSS cytopenias, karyotypes and risk group, mRNA levels of ABAT, and methylation percentages were all associated with OS of patients with MDS, whereas age, marrow blast count and the degree of ABAT methylation were independent factors for the survival of patients with MDS. Biological function analysis revealed that cell apoptosis and cell cycle distribution were closely associated with ABAT gene expression. Future studies should investigate the role of the ABAT gene in the molecular mechanisms in patients with MDS. In the future, molecular inhibitors may be designed that target the TCA cycle, in order to offset the function of the ABAT gene in leukemia, or that can be combined with other inhibitors to provide possible strategies for clinical treatment.
Acknowledgments
The authors would like to thank Dr Yan Wang (Huashan Hospital, Fudan University) for his operation and analysis of flow cytometry.
Funding
The present study was supported by grants from The Natural Science Foundation of Shanghai (grant nos. 16ZR1404400 and 17ZR1403600) and The Key Construction Building Subject of Three-year Action Plan of Fourth Round of Public Health Project in Shanghai (grant no. 15GWZK0801).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JG and XW designed the experiments. GZ performed and analyzed most of the experiments, and wrote the manuscript. SL and WW collected and analyzed MDS patient blood samples. NL performed some experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study was approved by the Ethics Committee of Huashan Hospital, Fudan University (approval no. KY2015-269). Patients provided informed consent.
Patient consent for publication
Informed consent was obtained from all individual participant included in the study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.del Rey M, O’Hagan K, Dellett M, Aibar S, Colyer HA, Alonso ME, Díez-Campelo M, Armstrong RN, Sharpe DJ, Gutiérrez NC, et al. Genome-wide profiling of methylation identifies novel targets with aberrant hypermethylation and reduced expression in low-risk myelodysplastic syndromes. Leukemia. 2013;27:610–618. doi: 10.1038/leu.2012.253. [DOI] [PubMed] [Google Scholar]
- 2.Leone G, Voso MT, Teofili L, Lübbert M. Inhibitors of DNA methylation in the treatment of hematological malignancies and MDS. Clin Immunol. 2003;109:89–102. doi: 10.1016/S1521-6616(03)00207-9. [DOI] [PubMed] [Google Scholar]
- 3.Khan H, Vale C, Bhagat T, Verma A. Role of DNA methylation in the pathogenesis and treatment of myelodysplastic syndromes. Semin Hematol. 2013;50:16–37. doi: 10.1053/j.seminhematol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Yang F, Li S, Liu M, Ying S, Jia X, Wang X. CpG island methylator phenotype of myelodysplastic syndrome identified through genome-wide profiling of DNA methylation and gene expression. Br J Haematol. 2014;165:649–658. doi: 10.1111/bjh.12811. [DOI] [PubMed] [Google Scholar]
- 5.Dexheimer GM, Alves J, Reckziegel L, Lazzaretti G, Abujamra AL. DNA methylation events as markers for diagnosis and management of acute myeloid leukemia and myelodysplastic syndrome. Dis Markers. 2017;2017;5472893 doi: 10.1155/2017/5472893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Chen Q, Gu J, Li S, Zhao G, Wang W, Wang Z, Wang X. Synergistic inhibitory effects of deferasirox in combination with decitabine on leukemia cell lines SKM-1, THP-1, and K-562. Oncotarget. 2017;8:36517–36530. doi: 10.18632/oncotarget.16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis. 2013;33:1073–1088. doi: 10.3233/JAD-2012-121330. [DOI] [PubMed] [Google Scholar]
- 8.Besse A, Wu P, Bruni F, Donti T, Graham BH, Craigen WJ, McFarland R, Moretti P, Lalani S, Scott KL, et al. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab. 2015;21:417–427. doi: 10.1016/j.cmet.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budczies J, Brockmöller SF, Müller BM, Barupal DK, Richter-Ehrenstein C, Kleine-Tebbe A, Griffin JL, Orešič M, Dietel M, Denkert C, et al. Comparative metabolomics of estrogen receptor positive and estrogen receptor negative breast cancer: Alterations in glutamine and beta-alanine metabolism. J Proteomics. 2013;94:279–288. doi: 10.1016/j.jprot.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Jansen MP, Sas L, Sieuwerts AM, Van Cauwenberghe C, Ramirez-Ardila D, Look M, Ruigrok-Ritstier K, Finetti P, Bertucci F, Timmermans MM, et al. Decreased expression of ABAT and STC2 hallmarks ER-positive inflammatory breast cancer and endocrine therapy resistance in advanced disease. Mol Oncol. 2015;9:1218–1233. doi: 10.1016/j.molonc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Wang F, Feng J, Zhou R, Chang Y, Liu J, Zhao Q. Co-expression network analysis identified six hub genes in association with metastasis risk and prognosis in hepatocellular carcinoma. Oncotarget. 2017;8:48948–48958. doi: 10.18632/oncotarget.16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett JM. World Health Organization classification of the acute leukemias and myelodysplastic syndrome. Int J Hematol. 2000;72:131–133. [PubMed] [Google Scholar]
- 13.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Besse A, Petersen AK, Hunter JV, Appadurai V, Lalani SR, Bonnen PE. Personalized medicine approach confirms a milder case of ABAT deficiency. Mol Brain. 2016;9:93. doi: 10.1186/s13041-016-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Yao DM, Qian J, Wang YL, Han LX, Jiang YW, Fei X, Cen JN, Chen ZX. Methylation status of fragile histidine triad (FHIT) gene and its clinical impact on prognosis of patients with myelodysplastic syndrome. Leuk Res. 2008;32:1541–1545. doi: 10.1016/j.leukres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Iwai M, Kiyoi H, Ozeki K, Kinoshita T, Emi N, Ohno R, Naoe T. Expression and methylation status of the FHIT gene in acute myeloid leukemia and myelodysplastic syndrome. Leukemia. 2005;19:1367–1375. doi: 10.1038/sj.leu.2403805. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Wang XQ, Xu XP, Lin GW. ID4 methylation predicts high risk of leukemic transformation in patients with myelodysplastic syndrome. Leuk Res. 2010;34:598–604. doi: 10.1016/j.leukres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Wu SJ, Yao M, Chou WC, Tang JL, Chen CY, Ko BS, Huang SY, Tsay W, Chen YC, Shen MC, et al. Clinical implications of SOCS1 methylation in myelodysplastic syndrome. Br J Haematol. 2006;135:317–323. doi: 10.1111/j.1365-2141.2006.06293.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou JD, Lin J, Zhang TJ, Ma JC, Yang L, Wen XM, Guo H, Yang J, Deng ZQ, Qian J. GPX3 methylation in bone marrow predicts adverse prognosis and leukemia transformation in myelodysplastic syndrome. Cancer Med. 2017;6:267–274. doi: 10.1002/cam4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo X, Nomdedeu M, Navarro A, Tejero R, Costa D, Muñoz C, Pereira A, Peña O, Risueño RM, Monzó M, et al. High levels of global DNA methylation are an independent adverse prognostic factor in a series of 90 patients with de novo myelodysplastic syndrome. Leuk Res. 2014;38:874–881. doi: 10.1016/j.leukres.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Tien HF, Tang JH, Tsay W, Liu MC, Lee FY, Wang CH, Chen YC, Shen MC. Methylation of the p15(INK4B) gene in myelodysplastic syndrome: It can be detected early at diagnosis or during disease progression and is highly associated with leukaemic transformation. Br J Haematol. 2001;112:148–154. doi: 10.1046/j.1365-2141.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 23.Dan C, Chi J, Wang L. Molecular mechanisms of the progression of myelodysplastic syndrome to secondary acute myeloid leukaemia and implication for therapy. Ann Med. 2015;47:209–217. doi: 10.3109/07853890.2015.1009156. [DOI] [PubMed] [Google Scholar]
- 24.Woods BA, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Immunol Rev. 2015;263:22–35. doi: 10.1111/imr.12246. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Menachem E. Mechanism of action of vigabatrin: Correcting misperceptions. Acta Neurol Scand Suppl. 2011;192:5–15. doi: 10.1111/j.1600-0404.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- 26.Maguire SE, Rhoades S, Chen WF, Sengupta A, Yue Z, Lim JC, Mitchell CH, Weljie AM, Sehgal A. Independent effects of gamma-Aminobutyric acid transaminase (GABAT) on metabolic and sleep homeostasis. J Biol Chem. 2015;290:20407–20416. doi: 10.1074/jbc.M114.602276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiNardo CD, Jabbour E, Ravandi F, Takahashi K, Daver N, Routbort M, Patel KP, Brandt M, Pierce S, Kantarjian H, et al. IDH1 and IDH2 mutations in myelodysplastic syndromes and role in disease progression. Leukemia. 2016;30:980–984. doi: 10.1038/leu.2015.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louro P, Ramos L, Robalo C, Cancelinha C, Dinis A, Veiga R, Pina R, Rebelo O, Pop A, Diogo L, et al. Phenotyping GABA transaminase deficiency: A case description and literature review. J Inherit Metab Dis. 2016;39:743–747. doi: 10.1007/s10545-016-9951-z. [DOI] [PubMed] [Google Scholar]
- 29.Barnby G, Abbott A, Sykes N, Morris A, Weeks DE, Mott R, Lamb J, Bailey AJ, Monaco AP, International Molecular Genetics Study of Autism Consortium Candidate-gene screening and association analysis at the autism-susceptibility locus on chromosome 16p: Evidence of association at GRIN2A and ABAT. Am J Hum Genet. 2005;76:950–966. doi: 10.1086/430454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jirholt J, Asling B, Hammond P, Davidson G, Knutsson M, Walentinsson A, Jensen JM, Lehmann A, Agreus L, Lagerström-Fermer M. 4-aminobutyrate aminotransferase (ABAT): Genetic and pharmacological evidence for an involvement in gastro esophageal reflux disease. PLoS One. 2011;6:e19095. doi: 10.1371/journal.pone.0019095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegerer M, Adena S, Pfennig A, Czamara D, Sailer U, Bettecken T, Müller-Myhsok B, Modell S, Ising M. Variants within the GABA transaminase (ABAT) gene region are associated with somatosensory evoked EEG potentials in families at high risk for affective disorders. Psychol Med. 2013;43:1207–1217. doi: 10.1017/S0033291711002923. [DOI] [PubMed] [Google Scholar]
- 32.Musialik E, Bujko M, Kober P, Grygorowicz MA, Libura M, Przestrzelska M, Juszczyński P, Borg K, Florek I, Jakóbczyk M, et al. Comparison of promoter DNA methylation and expression levels of genes encoding CCAAT/enhancer binding proteins in AML patients. Leuk Res. 2014;38:850–856. doi: 10.1016/j.leukres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Bert SA, Robinson MD, Strbenac D, Statham AL, Song JZ, Hulf T, Sutherland RL, Coolen MW, Stirzaker C, Clark SJ. Regional activation of the cancer genome by long-range epigenetic remodeling. Cancer Cell. 2013;23:9–22. doi: 10.1016/j.ccr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee SM, Choi WY, Lee J, Kim YJ. The regulatory mechanisms of intragenic DNA methylation. Epigenomics. 2015;7:527–531. doi: 10.2217/epi.15.38. [DOI] [PubMed] [Google Scholar]
- 35.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol. 2013;20:274–281. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- 36.Ouzounoglou E, Dionysiou D, Stamatakos GS. Differentiation resistance through altered retinoblastoma protein function in acute lymphoblastic leukemia: In silico modeling of the deregulations in the G1/S restriction point pathway. BMC Syst Biol. 2016;10:23. doi: 10.1186/s12918-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Bonelli P, Tuccillo FM, Borrelli A, Schiattarella A, Buonaguro FM. CDK/CCN and CDKI alterations for cancer prognosis and therapeutic predictivity. BioMed Res Int. 2014;2014;361020 doi: 10.1155/2014/361020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies C, Hogarth LA, Dietrich PA, Bachmann PS, Mackenzie KL, Hall AG, Lock RB. p53-independent epigenetic repression of the p21(WAF1) gene in T-cell acute lymphoblastic leukemia. J Biol Chem. 2011;286:37639–37650. doi: 10.1074/jbc.M111.272336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.