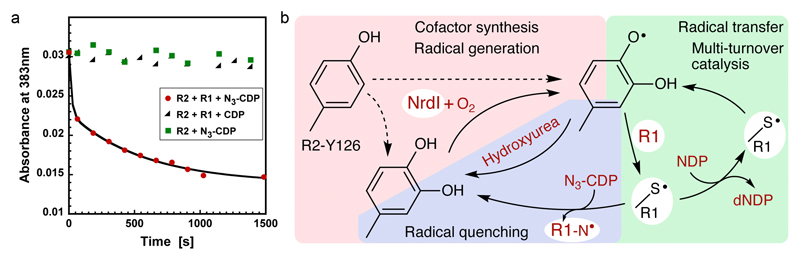
Fig 4. Catalytic competency of the radical and proposed mechanistic scheme in class Ie RNR.
a) UV/vis absorbance at 383 nm vs time. The radical signal is quenched in the presence of protein R1 and the mechanism-based inhibitor N3-CDP (red). Protein R2 with N3-CDP alone (green) or turnover conditions with protein R1 and CDP (black) does not quench the MfR2 radical. The experiment shows that the observed radical can be reversibly transferred to the active site and support catalysis in protein R1. Experiments were repeated three times.
b) Proposed mechanistic steps in class Ie RNR. The radical-harboring cofactor is first post-translationally generated by hydroxylation of Tyr126 in an NrdI and O2 dependent process. Dashed lines indicate alternative paths for the post-translational modification of Tyr126. It is presently unknown if this reaction also directly forms the radical species. Once the DOPA radical is formed in protein R2 it supports multi-turnover ribonucleotide reduction together with protein R1, presumably analogous to other class I RNR systems. If the radical is lost, activity can be restored in the covalently modified R2 protein by NrdI, again in an O2 dependent process.