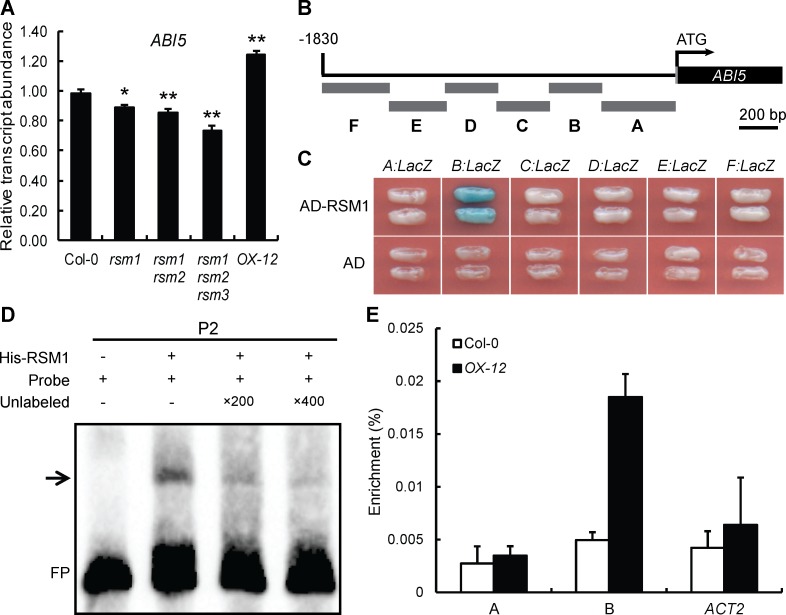
Fig 4. RSM1 binds to the promoter of ABI5 to regulate its expression.
(A) Expression of ABI5 in 1-day-old germinating seeds (Col-0, rsm1, rsm1 rsm2, rsm1 rsm2 rsm3 and OX-12) grown on MS medium as determined by qRT-PCR. The relative transcript levels were normalized to the abundance of reference gene ACT2 and shown as the means ± SD of three replicates (n = 3). The data are shown as the mean ± SD of three replicates per experiment (* p<0.05 and ** p<0.01). (B) Diagram of the ABI5 promoter fragments used to drive LacZ reporter gene expression in the yeast one-hybrid assays shown in (C). (C) Yeast one-hybrid assay, showing that RSM1 binds to fragment B of the ABI5 promoter. EGY48 cells were co-transformed with pB42AD-RSM1 and the pLacZ2U-ABI5 promoter. pB42AD was used as a negative control. (D) EMSAs showing that RSM1 binds to subfragment P2 of the ABI5 promoter. The symbols “+” and “−” indicate presence or absence respectively, of the reagent shown at the top left of the panel. FP, free probe. (E) ChIP-qPCR to assess RSM1 binding to the ABI5 promoter. Twelve-day-old WT and OX-12 seedlings were harvested for ChIP-qPCR assays using anti-RSM1 polyclonal antibody to immunoprecipitate RSM1 and its associated genomic DNA segments. The enrichment (%) was normalized to the level of input DNA. ACT2 and fragment A of the ABI5 promoter were used as negative controls. The data are shown as the mean ± SD from three independent replicate measurements (n = 3).