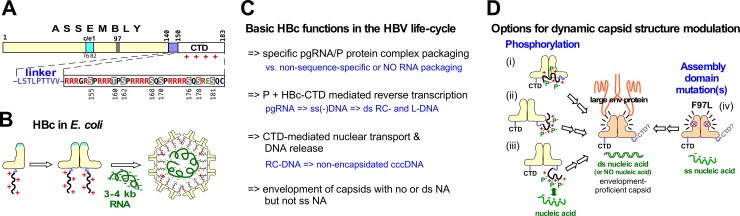
Fig 1. Structural and functional aspects of HBc.
(A) Domain organization. Numbers refer to aa positions. The immunodominant c/e1 epitope, in the 3D structure at the tips of the capsid spikes, and mutation F97L are indicated. Residues 141–149 (blue) link the assembly domain to the CTD which encompasses 16 basic R residues (red) and one acidic E residue (green). Phosphorylation of the S/T residues would reduce positive charge. (B) Non-sequence-specific RNA packaging by HBc in E. coli. HBc forms dimers that spontaneously assemble into CLPs. With HBc183, the basic CTDs mediate encapsidation of ~3–4 kb non-sequence-specific RNA. In HBV infection this would compete with specific encapsidation of viral pgRNA. (C) Basic functions of HBc in the HBV life-cycle. HBc is required to interact with different forms of viral genomic nucleic acid (NA) while avoiding interactions with irrelevant NAs. Progeny virus particles leaving a cell must be environmentally stable, yet upon infection of a new cell they must orderly release the viral NA for nuclear cccDNA formation. This implies temporal changes in NA binding capacity and capsid structure, including CTD disposition. (D) Options for capsid structure modulation. Regulated structure modulation is manifest by the selective, L protein dependent envelopment of dsDNA (or no NA) containing capsids (symbolized by the altered shape and color of the HBc dimer in the center) but not of capsids harboring RNA or ssDNA. Sensing the type of internal NA must involve the CTDs, and likely their phosphorylation status. The negatively charged phosphoryl groups could directly affect CTD—assembly domain interactions (i) and/or CTD disposition (ii), or act indirectly through altered NA binding (iii). Mutations like F97L evade this dependency, possibly by a priori promoting an envelopment-proficient structure.