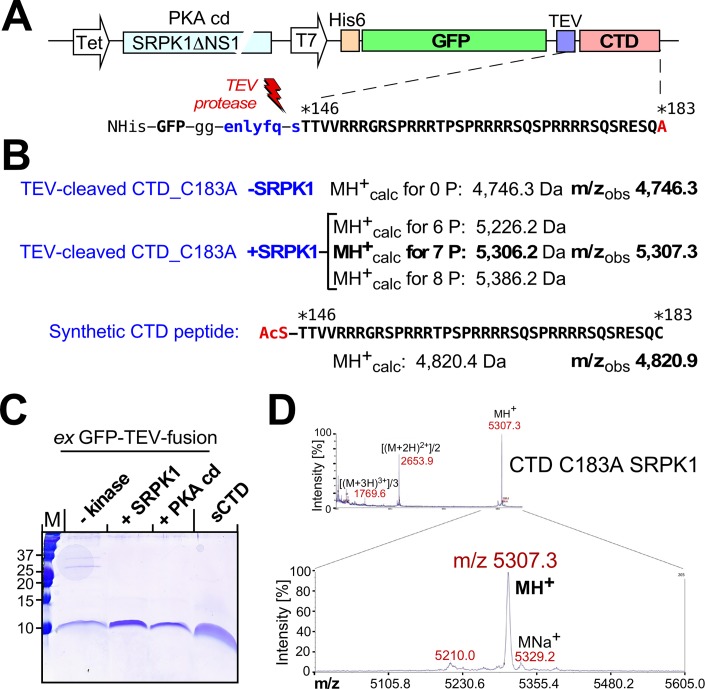
Fig 4. Accurate MS confirmation of seven SRPK1 phosphorylation sites in the HBc CTD.
(A) Genetic structure of the non-assembling CTD fusion constructs. The dual promoter vectors carried an ORF for non-His-tag kinase (SRPK1ΔNS1, or the catalytic domain of PKA) plus an ORF for a His-tagged GFP protein to which the CTD of HBc variant C183A was linked via a TEV protease recognition site; see text and S1 Protocol for details. (B) Calculated MH+ masses of the indicated CTD derivatives vs. observed m/z values. Note the excellent agreement for a seven-fold phosphorylated CTD species in the +SRPK1 sample. (C) SDS-PAGE of the purified CTD peptides from the indicated GFP fusion proteins. The sequence of the chemically synthesized N-acetylated sCTD peptide carrying the genuine C183 residue is shown in (B). (D) MALDI-TOF spectrum of the +SRPK1 CTD sample from (C). Note the predominance of the m/z peak corresponding to seven-fold phosphorylated CTD. Mass spectra for the other CTD peptides are shown in S6 Fig.