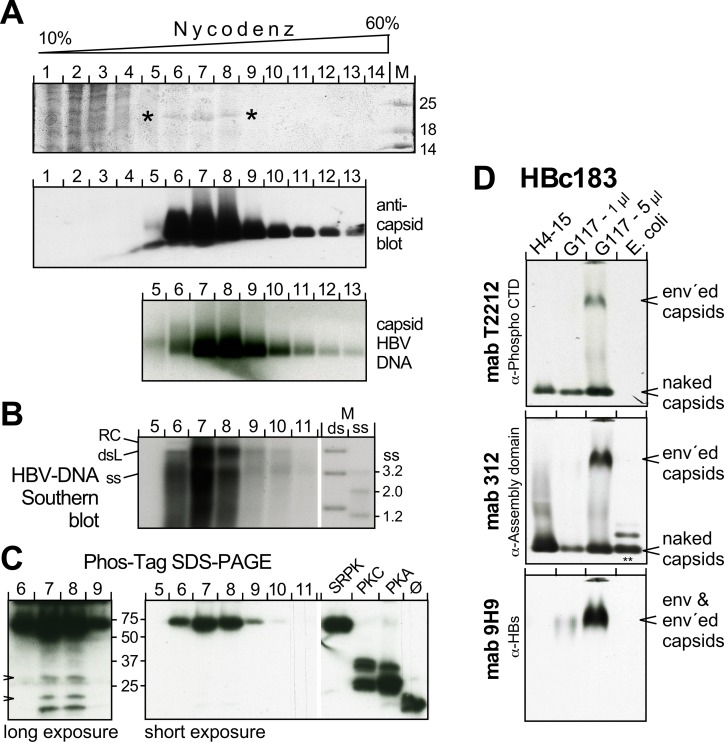
Fig 9. The bulk of HBc183 in capsids from human hepatoma cells is highly phosphorylated.
(A) Enrichment of particulate HBc by Nycodenz gradient sedimentation. Cytoplasmic lysate from HBV producing HepG2.117 cells was sedimented through a Nycodenz gradient. Fractions were analyzed by SDS-PAGE and CB staining (top panel; asterisks mark a 21 kDa band possibly representing HBc183; the complete gel is shown in S12A Fig); by NAGE followed by immunoblotting with the anti-HBc assembly domain mAb 312 as PO conjugate (middle panel); and by hybridization with a 32P-labeled HBV probe. (B) Southern blot for capsid-borne HBV DNA in individual gradient fractions. M, DNA marker comprising HBV-specific fragments of the indicated sizes loaded in native ds form, or in heat-denatured ss form. (C) Phos-Tag SDS-PAGE immunoblot. Aliquots from the respective gradient fractions and recombinant HBc183 coexpressed with the indicated kinases, or not (ø), were separated by Phos-Tag SDS-PAGE and immuno-blotted with mAb 1D8. Short exposure showed one band with comparably strong retardation as SRPK1-phosphorylated HBc183. Longer exposure (left panel) revealed weak additional bands with mobilities similar to those of unmodified and PKC and PKA phosphorylated HBc183 (arrowheads). (D) Enveloped capsids contain phosphorylated HBc183. PEG-precipitated particles in supernatants from HepG2.117 cells, or from an HBc183 producing Huh7 line, H4-15 were analyzed by NAGE immunoblotting alongside E. coli HBc183 CLPs. The blot was sequentially probed with mAb T2212 (anti-phospho-CTD), mAb 312, and mAb 9H9 (anti-HBs). Note that T2212 detected only HBc from eukaryotic cells, including a low mobility species that comigrated with HBsAg and was absent from the H4-15 samples; it therefore represents enveloped capsids. Additional data employing mAb T2212 are presented in S12 Fig.